Author(s): <p>Narula Ajit Singh, Sanjeev Gulati, Azmat Karim* and Himanshu Saini</p>
Minoxidil is a medication used to treat hypertension by directly dilating the arteries. However, about 3% of patients who take this medication experience pericardial effusion, which can lead to large pleural effusions and may require pleuro pericardiocentesis. In rare cases, patients may develop seroconstrictive pericarditis. This report describes an elderly woman who developed seroconstrictive pericardial effusion and bilateral pleural effusion seven months after taking minoxidil therapy. It has also been reported that minoxidil used in dialysis patients can cause large-volume pleuro pericardial effusions, leading to seroconstrictive pericardial effusion and anasarca, which is not common. Therefore, if a patient is on dialysis and minoxidil and they have a pleural or pericardial effusion that is unresponsive to ultrafiltration, there is a possibility of minoxidil-associated polyserositis. It is important for healthcare providers to be aware of this potential adverse effect, as prompt discontinuation of the drug can save lives.
Minoxidil is a medication that directly dilates the arteries and is used to treat hypertension. Approximately 3% of patients taking it experience pericardial effusion, which can lead to large pleural effusions and could require pleuro pericardiocentesis. Although rare, some patients could develop seroconstrictive pericarditis [1]. This report describes the case of an elderly woman who developed seroconstrictive pericardial effusion and bilateral pleural effusion seven months after taking minoxidil therapy. The pleural effusions and pericardial constriction resolved within 2 months of discontinuing the medication.
A 54-year-old lady with end-stage kidney disease has been on maintenance hemodialysis three times a week for over a year. She presented shortness of breath on accustomed exertion and swelling over her face and legs for the last few months.
She gained around 10 kg of body weight and noticed a gradual decrease in urine volume to 50-100 ml per day. Clinical examination showed: weight 94 kg (dry weight 84 kg), blood pressure 160/100 mm Hg, heart rate 78/minute, pitting edema up to mid-thighs, raised Jugular venous pressure (JVP), no pallor or jaundice, Kussmaul’s sign negative.
During the examination, the patient’s respiratory system showed reduced breath sounds in the axillary and lower interscapular areas. Normal heart sounds were present, and the patient had ascites without hepatosplenomegaly. The following investigations were conducted: Haemoglobin level was 11.5gm/dl, total leucocyte count was 11500/mm3 with 74% neutrophils, serum creatinine was 8.8mg/dl, blood urea nitrogen (BUN) was 72mg/ dl, sodium was 132meq/L, potassium was 4.2meq/L, uric acid was 4.2meq/L, calcium was 8.8mg/dl, phosphorous was 3.2mg/dl, intact parathyroid hormone (iPTH) was 135pg/ml, bilirubin was 0.82mg/dl, alanine aminotransferase/aspartate aminotransferase was 18/22IU/L, alkaline phosphatase was 234IU/L, Gammaglutamyl transferase was 62U/L, lactate dehydrogenase (LDH) 220 mg/dl, high sensitivity cardiac Troponin was 8ng/L, and creatine phosphokinase-myocardial band (CPK-MB) was 4IU/L. The end titer of antinuclear antibody (ANA) was 1:40, and C3/C4 were within the normal range.
A 12-lead surface electrocardiogram revealed that the patient had left ventricular hypertrophy. X-rays of the chest showed that there were pleural effusions present in both lungs, and an ultrasound of the chest indicated that there was about 1 liter of pleural fluid inthe left pleural cavity and about 400 ml in the right pleural cavity. Echocardiography revealed that the inferior vena cava was dilated, and there was significant pericardial effusion with stranding, jerky septal motion, and significant respiratory variation across the mitral valve and tricuspid valves suggestive of the influence of the effusion on hemodynamics (Fig 1 and 2). Contrast-enhanced computerized tomography (CECT) chest (Fig 2) showed that there were circumferential pericardial effusions measuring 1.2 cm, 1.4 cm, and 2.5 cm along the heart’s inferior, lateral, and posterior surface, with pericardial thickening, bilateral pleural effusions, and underlying atelectasis.
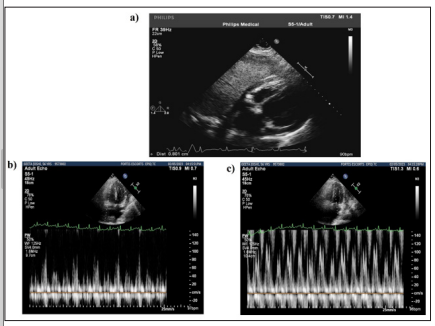
Figure 1: a, Echocardiography Doppler. Apical 4 chamber view showing Pericardial Effusion with stranding. b,c -Echocardiography showing significant respiratory variation across the tricuspid valve and mitral valves
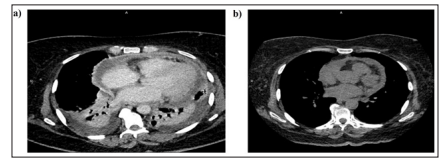
Figure 2: a. CECT-Chest Showing Pericardial Effusion with Thickening of Pericardium. Bilateral Pleural Effusions with Basal Atelectasis. b . CT Chest Showing Resolution of Pleural Effusions
The patient underwent a diagnostic pericardiocentesis and therapeutic left pleurocentesis. The analysis of the pleural fluid showed the following results: proteins - 3.2 gms/dl, sugar - 85 mg%, LDH 174 mg%, glucose - 285 mg/dl, adenosine deaminase - 14 units/L, and 800 cells/mm3 out of which 60% were lymphocytes. The smears for acid-fast bacillus (AFB), Grocott-Gomori’s methenamine silver stain (GMS) stain, and cultures were all negative, and the Gene-Xpert test for MTB was also negative. On the other hand, the analysis of the pericardial fluid revealed the following results: proteins - 2.2 gms/dl, sugar - 96 mg%, LDH 202 mg/dl, 100 cells/mm3, out of which 40% were lymphocytes. Cytology for malignant cells was negative, and the smears for AFB, GMS stain, and cultures were also negative. Moreover, the Gene-Xpert test for mycobacterium tuberculosis (MTB) was negative.
Despite daily hemodialysis for about 2 weeks and a reduction in about 5kg weight, she remained breathless. An ultrasound chest showed about a liter of fluid in the left pleural cavity, for which an intercostal drain was inserted. Her pericardial effusion persisted. A review of her drug chart showed she was on minoxidil for the last seven months. Minoxidil was stopped, and over the next week, the pleural fluid quantity was reduced to 50 ml per day, and the intercostal drain was removed with no re-accumulation of fluid. Two months later, a check X-ray of the chest and echocardiography showed complete resolution of the pleura and pericardial effusion.
At the onset, we were uncertain about the cause of the pleura pericardial effusion in our patient. We excluded malignancy, bacterial/tubercular infections, myocardial infarction, and autoimmune disease as potential causes. Uraemia was unlikely as the patient was regularly undergoing dialysis. We considered dialysis-associated effusions, as many patients with dialysisrelated pericarditis are known to improve with intensified dialysis. However, our patient did not improve, leading us to suspect a link between minoxidil and polyserositis. After stopping minoxidil, there was a significant regression of the pleura pericardial effusion over two weeks. At two months, the pleuro pericardial effusion had completely regressed.
Minoxidil is a prodrug converted into minoxidil sulfate, which helps open the adenosine triphosphate-sensitive potassium channel in the vascular smooth muscle of the arteriolar, leading to vasodilatation [2]. In our patient, it was started as the fourth anti-hypertensive drug for resistant hypertension. However, it can cause peripheral edema (7%) and pericardial effusion (3%) as side effects. Pericardial effusion is when fluid builds up around the heart and can progress to tamponade. This side effect is common in patients with cardiac failure and kidney disease on dialysis [3]. The mechanism behind fluid retention combines dose-dependent activation of the renin-angiotensin axis, leading to increased aldosterone biosynthesis and fluid retention. Moreover, activating the potassium channel in the ascending limb of the nephron can lead to increased Na+/2Cl-/K+ co-transporter activity, increasing sodium and chloride reabsorption [4].
There have been some reports of minoxidil used in dialysis patients causing large-volume pleuro pericardial effusions, leading to seroconstrictive pericardial effusion and anasarca, which is not common5,6. However, in most cases, stopping minoxidil leads to the disappearance of effusions. Pericardiocentesis and pleural fluid drainage may be necessary if a patient is hemodynamically unstable [5-6]. Significant pericardial effusions could be detected months or years after initiation of therapy. To prevent this, some experts suggest scheduling echocardiograms every 3 to 6 months, especially during the early phase of minoxidil therapy [2, 7]. This is because effusions can accumulate insidiously, as seen in our patients. Finally, the effusion may take up to 3 months to resolve after stopping minoxidil.
Multiple medications can cause drug-induced pericarditis. However, it is still an uncommon occurrence of drug toxicity. In the past, drugs like procainamide, hydralazine, and isoniazid caused drug-induced lupus and associated serositis. But recently, cardiac toxicity, including pericarditis, has been recognized as a side-effect of checkpoint inhibitors such as ipilimumab and nivolumab [2, 8].
If a patient is on dialysis and minoxidil and they have a pleural or pericardial effusion that is unresponsive to ultrafiltration, there is a possibility of minoxidil-associated polyserositis. It isessential to be aware of this potential adverse effect, as prompt drug discontinuation can save lives.
