Author(s): Ashish Kumar Tiwari and Brij Bhushan Tewari*
Metal ions play an important role in biological systems. The importance of metal ions to essential functions of living systems and for wellbeing of living organisms is well known. Complexation reactions of α– aminobutyric acid with cadmium (II), iron (II) and zinc (II) have been studied in solution phase using paper ionophoretic technique. The present method is based upon the migration of a spot of metal ion on a paper strip at different pH values of background electrolyte containing 0.01M α -aminobutyric acid. A graph of pH against mobility was used to obtained information in the binary complexes and to calculate its stability constants. The logarithm stability constants of ML and ML2 complexes of α -aminobutyric acid were found to be (4.01 ± 0.02; 2.72 ± 0.01), (3.73 ± 0.03; 2.65 ± 0.09), (4.45 ± 0.02; 2.80 ± 0.03) for cadmium (II), iron (II) and zinc (II) complexes, respectively at ionic strength 0.1 molL-1 and a temperature of 35º C. Metal based drugs bioactivity can be increase by metal chelation, which in turn increase their absorbance and stability. Metal complexes can offer their action such as anti-inflammatory, anti-diabetic, antimicrobial, anticancer and ant thyroid compounds.
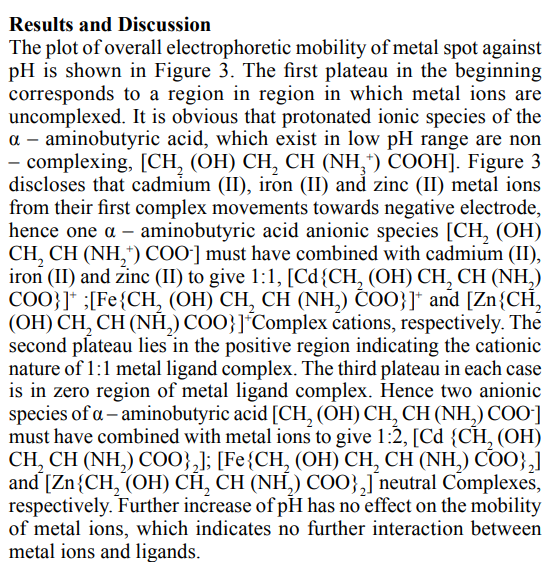
For the general case of complex MLn, the stepwise formation or stability constant (Ka) are:

Where M and L are metal cation and ligand anion, respectively. For the calculation of total concentration of final complex product (MLn), the overall formation constant is used.
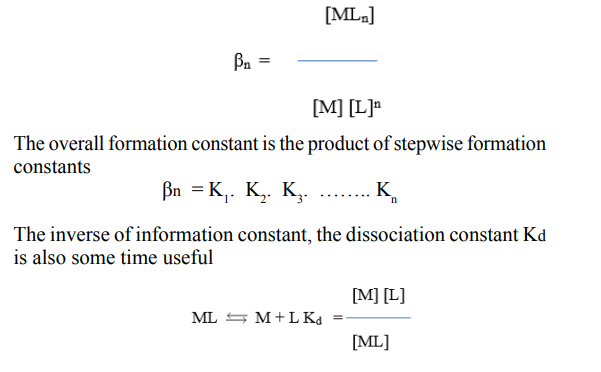
Kd has the same form as Ka for acids, which facilities comparisons between metal complexes and Bronsted acids. Out of all technique adopted for the study of metal ligand equilibria, we have opted for paper electrophoresis. Not much were, however, is on record on the application of paper electrophoresis for examining complexation reactions, metal complexes play an important role in various biological systems, hence the formation, stability, and reactivity of their complexes have been an active field of research [1].
Iron and zinc are classified as essential metals without which life cannot sustain. Cadmium is classified as toxic metal harmful even at low concentration. The average iron and zinc metal content of a healthy human (body weight 70 Kg) with plasma concentrations are 5.0 and 2.0 gram/man, respectively [2].
Heavy metals are defined as metals having a density higher than 5 g cm3. Cadmium has no known function as nutrients and seems to be more or less toxic to plants and microorganism. Concentrations of Cd ranged (0.04 nM – 0.32 nM) and (0.52 nM – 0.1 nM) in non – polluted and polluted soil, respectively [3]. Cd causes genotoxicity, teratogeny and cancer in some tissues and non malignent chronic toxicity in others (eg. Cancer in lung, nephrotoxicity in kidney) effect functions of neurotransmitters, hormones, and memory formation [4]. Cadmium has been shown to suppress iron uptake, induce phosphorus deficiency and reduce manganese transport in the growing plants [5].
Iron is important for electron transport in the respiratory chain and for various enzymatic reactions. Iron can harm biological systems since in redox – active form it catalyzes generation of highly reactive oxygen species. Both iron deficiency and overload impair cellular functions, the quantitation of iron in cells and extracellular fluids is of considerable interest [6]. Recent advances in genetics, molecular biology and biochemistry of iron metabolism have assisted in elucidating the molecular mechanism of iron homeostasis. Iron uptake and storage is tightly regulated by the feedback system of iron responsive element containing gene products and iron regulatory proteins that modulate the expression level of the genes involved in iron metabolism [7]. Zinc is an important trace element and essential for all living systems. Zinc is component in the metalloenzymes superoxide dismutase, alkaline phosphatase, alcohol dehydrogenase as well as RNA and DNA polymerase [8]. Zinc (II) complexes of α - amino acids and their derivatives with a Zn (N2 O2) coordination made were found to have in vitro insulinomimetic activity as estimated with the inhibition of free fatty acid release in isolated rat adipocytes treated with epinephrine. It was revealed that the insulinomimetic activities of zinc (II) complexes with overall stability constants (log β) less than 10.5 are higher than those of Zn SO4 and VO SO4 [9]. The studies in complexation reactions of bivalent cadmium, iron and zinc are of interest because of their nutrient properties and toxicity [10-18]. α – Aminobutyric acid is an isomer of the amino acid aminobutyric acid with chemical formula C4 H9 NO2.
It has two other isomers gamma – aminobutyric acid (GABA) and Beta – aminobutyric acid (BABA). It is a key intermediate in biosynthesis of ophthalmic acid or ophthalmate, which was first isolated from calf lens. GABA is one of the major inhibitory retinal neurotransmitters, and its localization has been established in nearly 40% of amacrine cells in all vertebrate’s species examined. There are three classes of GABA (GABAa, GABAb, GABAc) and all have been shown in retina [19]. α – Aminobutyric acid has significant applications in biological systems [20,21]. Kiso has done comprehensive study on paper electrophoretic migration of metal complexes [22].
The paper electrophoretic technique usually suffers from a number of defects, capillary flow on paper, electro – osmosis, temperature during electrophoresis and adsorption affect the mobility of charged moieties [23]. The present technique is almost free from these destroying factors and very convenient in use. It gives results in fair agreement with accepted literature values. Communications from our laboratory described a new method for the study of metal complexes [24-26].
A search of literature indicated few reports on zinc (II) - α – aminobutyric acid and iron (II) - α – aminobutyric acid complexes but no report available on cadmium (II) - α – aminobutyric acid complexes. In view of this, attempts were made to establish the optimum conditions for metal (II) - α – aminobutyric acid complex formation. In addition, present paper describes on paper ionophoretic method for the determination of the nature and stability constants of Cd (II)/Fe (II)/Zn (II) - α – aminobutyric acid complexes.
A systronic (Naroda, India) Model 604, electrophoresis was used. The apparatus consisted of a Poly (vinyl chloride) PVC moulded double tank vessel. In our laboratory significant change in the in the instrument has been made. Two hollow rectangular iron plates each weighing one kg, and covered with thin polythene sheets have been used through which thermostated water circulated for controlling the temperature. The tanks are closed with a transparent PVC moulded lid. The whole assembly is tight to prevent moisture change, which might upset the equilibria in the paper strip. This assembly design thus keeps to a minimum the disturbing effect of evaporation from the evaporation from the unwanted liquid flow in the paper strips. Each electrolyte tank contains a separate electrode chamber in which Pt-wire anode and cathode are placed, respectively. Applied voltage was from a stabilized source. Electrophoresis cell showing sandwiched paper strips is shown in Figure 1
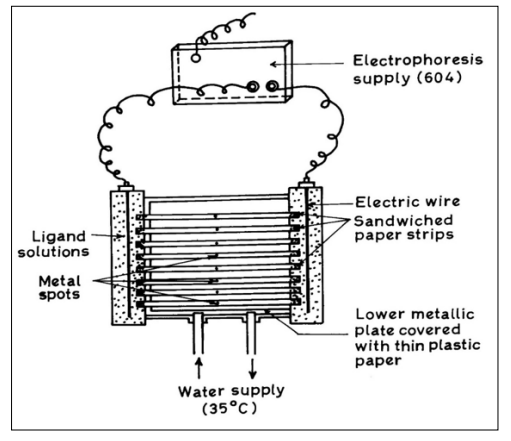
Figure 1: Electrophoresis Cell Showing Sandwiched Paper Strips
Whatman No 1 filter papers or chromatography were used for the purpose of electrophoresis. Elico (Hyderabad, India) Model L1-10 pH meter using a glass and calomel electrodes assembly working on 220 V/50 Hz established a. c. mains, was used for the pH measurements pH meter was calibrated with buffer solution of pH 7.0.
Cadmium (II), iron (II) and zinc (II) metal perchlorate solutions were prepared by the precipitation of metal carbonates from a 0.1 Mol L-1 solution of cadmium((II), iron (II) and zinc (II) nitrates with the solution of sodium carbonate (chemically pure grade, BDH, Poole, UK). The precipitation was washed with boiling water and treated with calculated amounts of 1% perchloric acid. The metal contents of the filtrates were determined, and final concentration was kept 0.005 Mol L-1.
Carbon dioxide free sodium hydroxide solution was prepared by dissolving 500 gms of sodium hydroxide in 500 mL of water in a flask. The flask was left overnight. The clear supernatant liquid was filtered rapidly using a high vacuum pump. A suitable volume of the filtrate was diluted, and the concentration determined by titrating against a standard oxalic acid solution. A solution (2.0 Mol L-1) was obtained by suitable dilution. The concentration of stock solution was checked from time to time.
Metal spots were detected on the paper using dithizone in carbon tetrachloride for Zn (II). A 0.1 % solution of 1- (2 – pyridylazo) – 2 – naphthol (PAN) (E. Merc, Darmstadt, Germany) in ethanol was used for detecting the cadmium (II) and iron (II) metal ion. A 0.005 Mol L-1 glucose BDH, Analytical Reagent grade solutions was prepared in water and used as an electro osmotic indicator for the correction due to electro – osmosis. A saturated aqueous solution (o.9 ml) of silver nitrate was diluted with acetone to 20 ml. Glucose was detected by spraying with this silver nitrate solution and then with 2% ethanolic solution of sodium hydroxide, when a black spot was formed. Paper strips showing the position of metal ions spot after electrophoresis is shown in Figure 2.
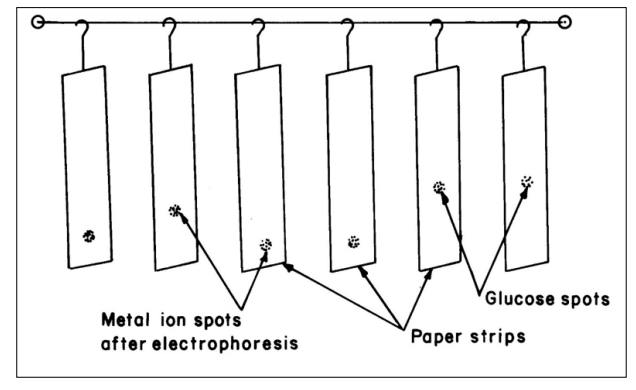
Figure 2: Paper Strips Showing Position of Metal Ion Spots After Electrophoresis
The background electrolytes used in the study of binary complexes were 0.1 Mol L-1 perchloric acid and 0.1 Mol L-1 α – aminobutyric acid. The system was maintained at various pH by the addition of sodium hydroxide. Stock solution of 5.0 Mol L-1 perchloric acid (SDS, Analytical Reagent grade), 2.0 Mol L-1 sodium hydroxide (Analytical Reagent grade) and 0.5 Mol L-1 α – aminobutyric acid was prepared. Each solution was standardized using the appropriate method.
Whatman No.1 filter paper chromatography was used for the purpose of electrophoresis. For recording observation of particular metal ion, two strips were spotted with the metal ion solution along with additional two spotted with glucose using 1.0 µL pipette and mounted on insulated plate. Each of the two-electrolyte vessels were filled with 150 mL of background electrolyte containing 0.1 Mol L-1 perchloric acid and 0.01 Mol L-1 α – aminobutyric acid. The paper became moistened with the background electrolyte solutions due to diffusion. The second insulated plate was placed on paper strips and then thermostated water (35º C) was circulated in the plates to keep the temperature constant. The lid was then placed on the instrument to make it airtight. It was left for 10 minutes to insure wetting the strips. Subsequently a direct 200 Volts potential was applied between the electrodes. Electrophoresis was carried for 60 minutes after which these strips were removed from the tank and dried. The metal ion and glucose spots were detected by specific reagents. The leading and tailing edge were measured from the marked center point and the mean were taken. The distance moved by glucose was subtracted (in case of migration toward anode) to obtain correct path length. Migration towards anode and cathode were designated by negative and positive signs, respectively.
Electrophoretic observations on metal ions were recorded at various pH values of the background electrolyte obtained by adding sodium hydroxide solution. The ionic strength being maintained at 0.1 Mol L-1. The observed mobility of migrants was calculated by using the formula.
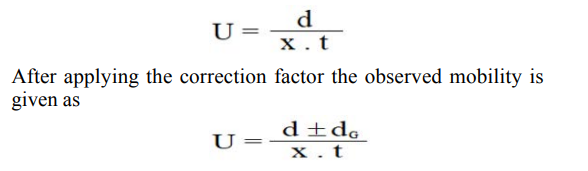
where U = mobility of metal ion / complex ion, d = mean of duplicate distance travelled by metal ion / complex ion; dG = mean duplicate distance travelled by glucose spot; x = field strength; t = time for electrophoresis. The mobility of metal / complex ion spots on the paper strips were thus calculated and are reported with different pH values Figure 3.
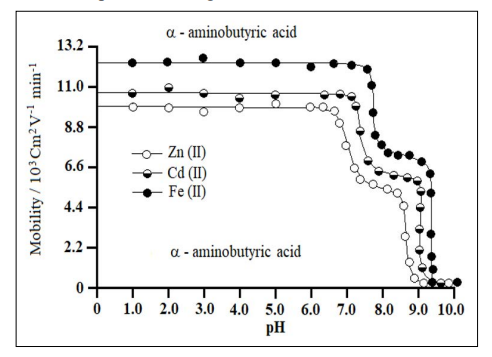
Figure 3: Mobility Curves for Metal (II) - α – Aminobutyric Acid Complexes
Background electrolytes = 0.1 Mol L-1 perchloric acid and 0.01 Mol L-1 α – aminobutyric acid. Concentration of metal ions = 0.005 Mol L-1. Variation in the pH was made by the addition of sodium hydroxide.
The protonation constants of pure α – aminobutyric acid was determined by the same paper electrophoresis technique. The two paper strips were spotted with α – aminobutyric acid along with two glucoses using 0.1 Mol L-1 perchloric acid only in a background electrolyte. The electrophoresis was carried for 60 minutes as for metal ion. The electrophoresis speed was calculated.
The speed of α – aminobutyric acid spots are reported with various pH values. The individual speeds of duplicate spots were found to be fairly equal.
Chemical literature also assigns prominent chelating properties of the unprotonated anionic species of α – aminobutyric acid ruling out any such property to zwitterions [27]. In general, the complexation of metal ions with α – aminobutyric acid anion [L-] may be represented as
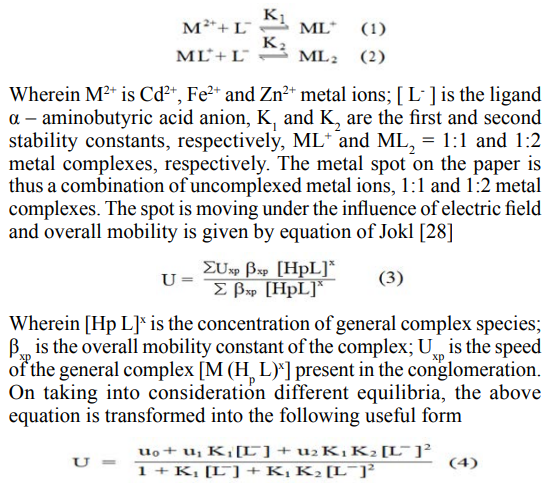
Wherein u0, u1 and u2 are mobilities of uncomplexed metal ion, 1:1 metal complex and 1:2 metal complex, respectively [28].
The protonation constants of pure α – aminobutyric acid (pk1 = 2.30; pk2 = 9.63) was obtained by the same paper ionophoretic technique the mode of dissociation of pure α – aminobutyric acid represented as
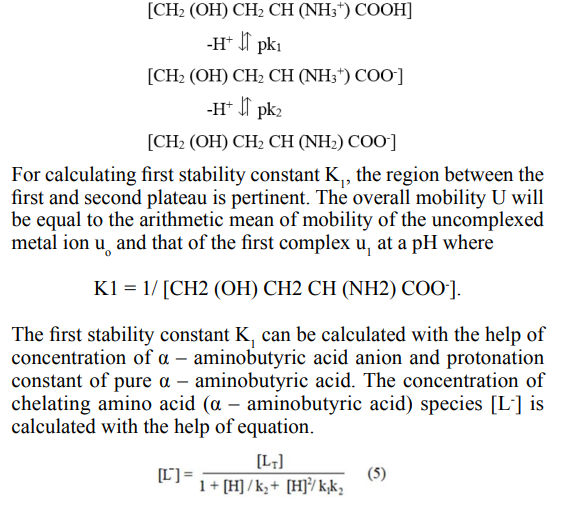
Where [L T] = is the total concentration of ligand (α – aminobutyric acid) 0.01Mol L-1. The second stability constant K2 of 1:2 metal ligand complex can be calculated by taking into consideration the region between the second and third plateau of the mobility curve. The calculated values of first and second stability constants are given in Table 1.
|
Metal ions |
Stability constants |
Complexes |
Logarithm stability calculated |
Constant values literature |
|
Cadmium |
K1 |
ML+ |
4.01 ± 0.02 |
- |
|
(II) |
K2 |
ML2 |
2.72 ± 0.01 |
- |
|
Iron (II) |
K1 |
ML+ |
3.73 ± 0.03 |
3.37 [29] |
|
K2 |
ML2 |
2.65 ± 0.09 |
- |
|
|
Zinc (II) |
K1 |
ML+ |
4.45 ± 0.02 |
4.54 ± 0.04 [29] |
|
K2 |
ML2 |
2.80 ± 0.03 |
4.11 [29] |
Iron strength = 0.1 Mol L-1; temperature 35º C; α – aminobutyric acid anion: [CH3 - CH2 CH (NH2 ) COO- ]; M= metal cations [Cd2+, Fe2+, Zn2+]; L = Ligand (α – aminobutyric acid); concentration of metal cation = 0.005 Mol L-1; pH of solution maintained by addition of sodium hydroxide. The paper strips were spotted with 0.1 µL of sample solution and glucose (for making osmotic correction)
It is clear from the Table 1 that first and second stability constants of cadmium (II), iron (II) and zinc (II)- α – aminobutyric acid complexes follow the order Log K1 > Log K2, the corresponding second stability constant values are found to be lower for all complexes. It is therefore inferred that coordinating tendency of a ligand decreases with the higher state of aggregation.
It is also clear from the Table 1 that first and second stability constants follow the order zinc (II) > cadmium (II) > iron (II). It is observed from Table 1 that the presented stability constant values are similar to literature values. The slight deviation in values obtained from different sources is mainly due to the difference in temperature and ionic strength used by different researchers.
The stability constants of metal complexes can be very easily calculated by this technique, therefore present method have significant advantages over other methods (viz: polarography, potentiometry, solubility etc.) reported in chemical literature for the determination of stability constants of metal complexes. According to standard deviation (statistics) the precision of method limited to that of paper electrophoresis, and uncertainty in the result is ± 5%. Hence, it cannot immediately replace the most reliable methods, even though it is new approach deserving further development.
The high stability constant values of zinc (II) - α – aminobutyric acid complexes indicating strong bonding between zinc (II) cation and α – aminobutyric acid anion. Whilst low stability constant values of iron (II)- α – aminobutyric acid complexes indicate weak bonding between iron (II) cation and α – aminobutyric acid anion. The higher stability of zinc (II) complexes may be due to its greater affinity for oxygen donor ligands.
The proposed structure for metal (II) - α – aminobutyric acid, ML2, binary complexes may be given as follows
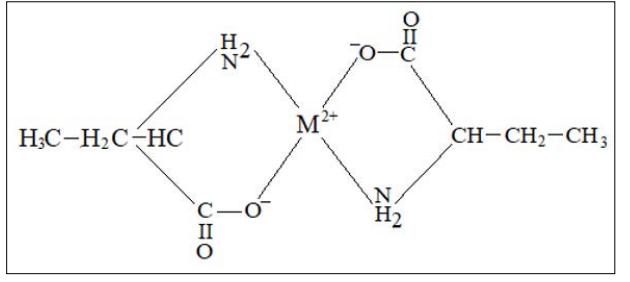
The following conclusions can be drawn from the present studies:
