Author(s): Luis Alejandro Boccalatte, Florencia Di Rocco, Rocio Bruballa*, Marcelo Figari and Juan Larrañaga
Solitary fibrous tumors (SFT) are rare entities that can arise from almost any tissue. The treatment of choice is always complete surgical resection, which may require major reconstructions. We report a case of a 49 year old man with a recurrent SFT of the orbit and anterior skull base, who underwent eight surgical procedures. A craniofacial resection was successfully performed. The reconstruction was made with an anterolateral thigh flap, and a titanium mesh. Management of these tumors is always challenging. They must be approached by a specialized multidisciplinary team, with adequate preoperative planning.
Solitary fibrous tumors (SFT) are a rare entity more frequently described in the pleura. Lately there have been multiple reports of this kind of tumor in the head and neck region, being the largest published series, the one of Memorial Hospital (MSKCCNY) in 2003 with 12 patients in a 15 year period [1-5]. SFT are neoplasms of mesenchymal origin that share so many histological characteristics with the hemangiopericytoma that some authors consider them to be the same tumor [6,7]. Most of these tumors show benign behaviour, however, there are malignant cases reported in literature. The diagnosis of malignancy is made based on certain histopathological characteristics such as increased cellularity, absence of alternating areas of sclerosis and a mitotic rate of at least 4 mitoses per 10 high-power fields (HPF) [5].
Due to their great vascularity these tumors are greatly enhanced with contrast in imaging studies such as Computed Tomography (CT) or Magnetic Resonance Image (MRI) [8]. The treatment of choice is complete surgical resection. Its survival rate is difficult to determine due to the low frequency of this type of tumors, but it is expected to be high when negative margins are achieved. Adjuvant therapies such as radiotherapy, chemotherapy and vascular inhibitors [6] are currently proposed as part of the treatment. We report a case of a multiple recurrent, previously irradiated SFT of the orbit with maxillary, cranial base and contralateral orbital involvement. We discuss the resective procedure, its complications, the complex multiple-stage reconstruction and the follow up of the patient.
A 49 year-old patient was referred to our department from another institution with a history of eight previous resection attempts of a hemangiopericytoma of the orbit and anterior skull base, all of which were approached through coronal incision and frontal craniotomy. The patient had also received full radiation treatment. During our first evaluation, the patient presented massive exophthalmos with total vision loss of the left eye (Figure 1A) and an expansive palatal mass. The revision of the pathology specimen at our institution informed a benign SFT.
CT-scan (Figure 1B) and MRI showed an invasive mass that enhanced with contrast and invaded the left orbit and maxilla, the anterior cranial base and the contralateral orbital wall. These studies also showed old sequelae of a cerebrovascular ischemic event.
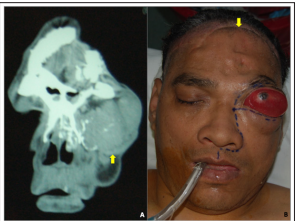
Figure 1: CT-scan and clinical examination A. CT-scan showed an invasive mass that enhanced with contrast and invaded the left orbit and maxilla, the anterior cranial base and the contralateral orbital wall. B. Massive exophthalmos with total vision loss of the left eye and an expansive palatal mass. The revision of the pathology specimen at our institution informed a benign SFT. The yellow arrow point out the previous coronal approach
Due to the history of multiple bleeding episodes during previous surgeries, an angiographic study was done; even though the study revealed a highly vascular tumor no major vessels could be individualized in order to be embolized. A craniofacial resection was performed through a coronal incision and a frontal craniotomy (the frontal bone was discarded due to gross compromise secondary to previous surgeries) The inferior side of the tumor was approached through a Weber-Ferguson incision with subciliary extension (Figure 2A). The surgical specimen included the ethmoid bone, the left anterior cranial base sparing the orbital rim, the medial right orbital wall, periorbital tissue and a radical maxillectomy with orbital resection (Figure 2B). All frozen margins were negative and the final pathology report informed a tumor constituted by fusiform cells with round and oval nuclei, scarce and slightly eosinophilic cytoplasm forming short fascicles with a delicate vascular network of an hemangiopericytoma type and fibrohyaline bands (Table 1).
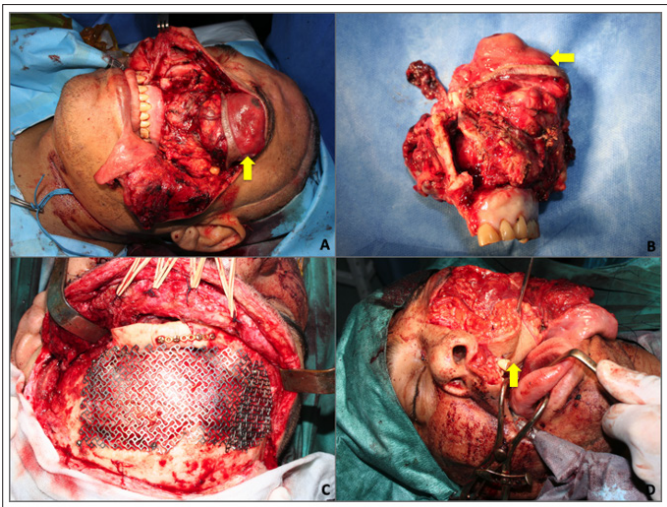
Figure 2: First surgery: Resection and reconstruction.
A. Weber Ferguson approach with subciliary extension was performed.
The yellow arrow delimits the tumor’s location.
B. Surgical specimen.
C. The anterior wall of frontal bone was reconstructed with
titanium mesh.
D. The orbit defect was reconstructed with an anterolateral tight microsurgical flap (yellow arrow).
| CD34 | + capillary and tumor cells |
| CD31 | + vascular walls - tumor cells |
| HHF35 | + capillary BM - tumor cells |
| CD117 | - |
| Mitotic index | low |
The resulting defect was reconstructed with an anterolateral thigh (ALT) flap with two skin paddles, the first one for the nasal cavity and the second one for the palate and a portion of vastus lateralis to seal the dura defect. An immediate cranioplasty was performed with a titanium mesh and the contralateral orbit was also reconstructed with this material (Figure 2C and D). One week after discharge the patient suffered an exposure of the titanium mesh due to ischemic damage of the highly mistreated coronal flap (Figure 3A). This problem was solved with the extraction of the foreign material followed by another ALT flap that involved most of the vastus lateralis and a small (2 x 2 cm) skin paddle (Figure 3B). Two saphenous vein grafts had to be obtained in order to achieve an appropriate pedicle in the neck (Figure 3C). The patient evolved favorably (Figure 3D).
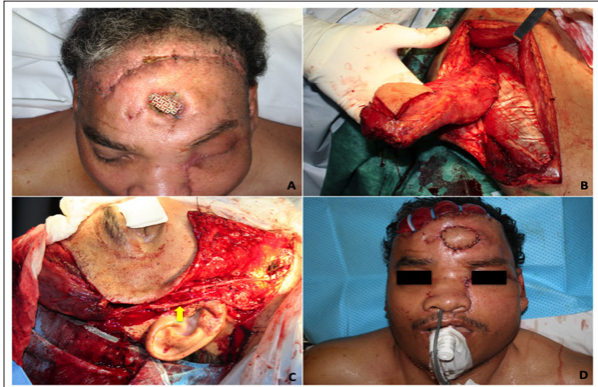
Figure 3: Second reconstructive time.
A. Titanium mesh exposure.
B. Anterolateral tight flap with skin paddle.
C. Reconstruction
time with microsurgical technique. The yellow arrow shows the saphenous vein grafts.
D. Final result.
One year after surgery, with imaging confirmation of no recurrence, we performed a cranioplasty with a custom made PEEK implant and also restored the left orbit with the same material (Figure 3A and B). A month later, the procedure was complemented with a free flap, this time from the radial forearm, to recreate the orbital cavity and palpebral fissure in order to fit a future prosthesis (Figure 3C). The orbital prosthesis suffered minimal exposure at the suture line but, as the wound presented some suspicious drainage, it had to be removed. The patient did not accept reconstructing this defect with a fibula flap in order to establish dental rehabilitation. The patient is now 10 years follow-up free of disease (Figure 4).
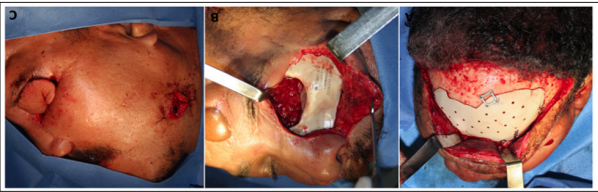
Figure 4: Third reconstructive time.
A and B. Orbit and frontal bone reconstruction with PEEK prosthesis.
C. Soft tissue
reconstruction with a radial forearm flap.
SFT are very rare tumors that more frequently appear in the pleura but there are several reports of this tumor being found in the head and neck region. Our patient had a SFT with origin in the orbit. We coincide with the literature available about the characteristics of this tumor, which was highly avid for contrast in both CT and MRI, and was first diagnosed as benign. Even though it eroded the bony structures of the skull and face, it kept a clear limit from healthy tissues [9,10]. The first pathology report informed a hemangiopericytoma and near total resections were not able to control its growth, even when combined with radiotherapy
In the absence of many facial structures, we combined alloplastic and biologic materials for the reconstruction. Special care must be taken to cover all non-vital materials with well-irrigated tissue. For this purpose, free flaps were our first choice. We believe that even in tumors with such extension as this one, large craniofacial resections are extremely valid and can provide adequate control of the disease. Although these tumors are highly vascularized, we could not find any potential vessels to embolize, and no major bleeding was found during surgery. The reconstruction of defects of this dimension and complexity are daring and must be faced with patience, knowing that they will probably require multiple interventions, but also that excellent results can be achieved. We faced many complications during the treatment of this patient but since they were attended promptly and aggressively, they did not jeopardise the final outcome.
There are no conflicts of interest. None of the authors has any direct or indirect commercial financial incentive associated with the publication of this paper. The author(s) received no financial support for the research, authorship, and/or publication of this article.
The authors certify that they have obtained all appropriate patientconsent forms. In the form the patient(s) has/have given his/her/ their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
