Author(s): Menka Kapil* and Rateesh Sareen
M Pneumoniae infection commonly presents with respiratory involvement. Extra pulmonary involvement is uncommon presentation. The case describes M Pneumoniae infection presenting as hemolytic anemia which on work up turned out to be cold agglutinin disease. The rare presentation of M Pneumoniae infection without pulmonary involvement makes the case report interesting .It is therefore prudent to rule out M Pneumoniae infection in patients with unexplained hemolytic anemia or having cold agglutinin disease.
Mycoplasma is smallest and simplest self replicating bacteria. Mycoplasma Pneumoniae infection are often asymptomatic and when symptomatic they characteristically involve upper and lower respiratory tracts [1]. The signs and symptoms caused by M pneumoniae infection can be classified as pulmonary, presenting with tracheobronchitis or pneumonia and extra pulmonary where it may present with involvement of central nervous system, gastrointestinal tract or hematological manifestations. The extra pulmonary involvement of M pneumoniae is less common and may precede or occur during or after pulmonary presentation, as discussed in literature by Walters et al [2]. M pneumoniae forms cold agglutinins that result in antibody mediated hemolysis in 10% of patients although severe hemolysis is exceptional [3- 5]. M pneumoniae was initially categorized as primary atypical pneumoniae. It was Eaton who demonstrated agglutinating red blood cells by antibodies directed against M pneumonia [6,7]. He isolated an unidentified filterable agent, which was subsequently named Mycoplasma after cultivation on cell free media in 1962. M pneumoniae infection is characterized by long convalescence phase which may extend for 4-6 weeks [8].
Mycoplasma has a coccus form in culture, elongated or filamentous forms may also occur. The filaments tend to produce branched mycelia structures; hence named Mycoplasma (mycelia- fungus, plasma- form) [9]. It reproduces by binary fission. M Pneumoniae has a unique attachment organelles and exhibit gliding motility. They have a fried egg colony shape on agar. The membrane protein, glycolipids and lipoglycans on cell surface are major antigenic determinants. The antibodies formed against these antigens inhibit growth & metabolism of the organism and in the presence of compliment protein can also lysis [10]. M pneumoniae infected individuals may develop auto antibodies including cold agglutinins reacting with erythrocyte antigen and other antibodies that tract against lymphocytes, smooth muscle cells and brain &lung antigens [11]. The host defense is varied; generally the first antibodies to develop are IgM class. IgG appears late in the convalescence phase. IgA antibodies seem to play role in host resistance [12].
We report a case of severe hemolytic anemia in an immunocompetent host in the absence of marked clinical evidence of pneumoniae.
A 45 year old male patient was admitted to the hospital with complaints of dizziness, palpitations since 7 days and fever, vomiting since 2 days. The patient was previously healthy and was not taking any other drugs. On physical examination, the patient was febrile with yellow discoloration of sclera. The vitals were; Blood pressure 120/80 mm of Hg, pulse rate 100/minute and temperature - 100°F. The rest of examination and personal history were unremarkable. The initial investigations showed hemoglobin level of 2.3 g/dl, mean cell volume (MCV) 97.6 fl, total leucocytes count 10,320 / mm3 , with neutrophils-92%, lymphocytes-6%, monocytes-4%, platelet count- 2,00,000 / mm3 and erythrocyte sedimentation rate (ESR) 57 mm/ hr. A peripheral blood smear showed leucocytosis and agglutination in red blood cells (RBC). RBC showed fragmentation and crenation. Hemophagocytosis by macrophages engulfing neutrophil was noted (Fig-1) Reticulocyte count was 3.5 %. Biochemical investigations showed Blood urea nitrogen ( BUN)- 39 mg/dl, Creatinine 1.2 mg/dl, SGOT- 60 IU, SGPT-37 IU, Total bilirubin- 10.9 mg/dl, direct bilirubin- 4.2 mg/ dl, total protein- 5.7 gm/dl, Albumin-3.1 gm/dl, globulin- 2.6 gm/ dl, A/G ratio- 1.19, Alkaline phosphatase 95 IU, Gamma GT- 36 & Uric Acid -16.3 mg/dl. The electrolytes were within normal limits, Sodium- 146mEq/L, Potassium- 5.2 mEq/L and chloride 110 mEq/L. The Serum vitamin B12 was > 1000 pg/L. The patient tested non reactive for HIV-1 7 2, Hepatitis B surface antigen, hepatitis C antibody, Ig M anti HbS. Urine examination revealed a reddish hemorrhagic appearance with 2-3 / hpf RBC, 5-6/ hpf WBC, occasional epithelial cells and absence of any cast, crystal or bacteria. Dengue NS1 and IgM were negative. Patient tested negative for scrub typhus & rapid malaria antigen test. The work up for hemolytic anemia was done which showed a normal osmotic fragility curve, normal G6PD enzyme activity with positive direct & indirect coomb’s tests. Anti nuclear antibody (ANA), anti Sm antibody and anti LKM antibody were negative. The presence of agglutination in peripheral blood smear, hemoglobinuria in urine and positive coomb’s test prompted us to look for cause of immune mediated hemolysis. Meanwhile bone marrow aspiration and biopsy showed erythroid hyperplasia with adequate megakaryocytes and normal granulocytic maturation (Fig-2,3). The Ultrasonography abdomen showed hepatosplenomegaly with altered eco texture with splenomegaly; congestive changes were seen in Inferior vena cava and hepatic vein.
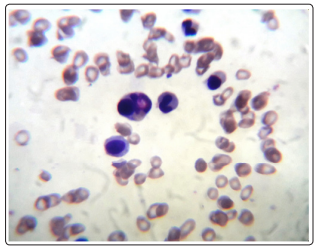
Figure 1: PBF ( 100 X oil) Leishmsn stain
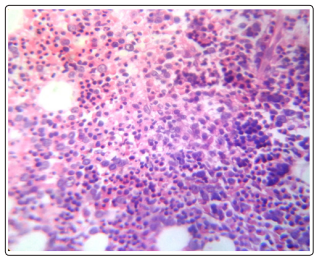
Figure 2: BMBx (40x) H & E
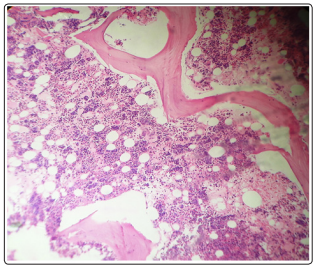
Figure 3: BMBx (10x) H & E
The case had hemoglobinuria, red cell agglutination on peripheral blood smear and positive coomb’s test (Direct & Indirect, both). A provisional diagnosis of immune mediated anemia was done. Further workup showed IgM positive antibody for M Pneumonia on immunofloresence.
The extra pulmonary manifestations are observed in 20-25% of patients infected with M Pneumonia [13]. Severe hemolysis as seen in our case is a rare occurrence and is generally associated with severe pulmonary involvement [4,14]. The IgM antibodies are directed against I antigen present on red blood cell membrane. This antibody producers a cold agglutinin in nearly half of the patients with M Pneumonia infection by activation of complement C3d resulting in positive coomb’s reaction [15,16]. A diagnosis of cold agglutinin hemolytic anemia (CAHA) was made. 50 -60% of patients with M Pneumonia have cold agglutinins disease [17]. The antibodies appear one week after onset of illness [4]. The cold agglutinins are formed against I antigen of red blood cell surface, Ig M antibody is seen in 90% of cases [18].
The exact mechanism of M Pneumonia infection caused cold agglutinin disease is not precisely known. The cells responsible for phagocytosis do not have receptor for IgM or IgG whereas the cold agglutinins are usually of Ig M class, it is believed that red blood cell destruction is due to activation of complement leading to membrane destruction or immunoadherance mediated hemolysis [19].
The CAHA is self limiting disease. In M Pneumonia infection antibiotics are given but they have limited use when associated with CAHA but at the same time their use is associated with quick resolution of hemolysis [20]. Packed red cell infusion in autoimmune hemolytic anemia can potentiate the hemolysis and therefore it is prudent to use transfusion in a limited manner as discussed by Gehrs and Friedburg [20,21]. One packed red cell unit was infused in our patient. After transfusion and antibiotic therapy with supportive care the patient improved.
M Pneumoniae rarely presents as hemolytic anemia without pulmonary involvement. It should always be kept in mind while dealing with unexplained hemolysis. The diagnosis requires peripheral blood smear examination, coomb’s test, complete urine examination and serum biochemistry with serological testing for M Pneumoniae.
Conflict of Interest: Nil
Sources of funding: Nil
