Author(s): Asma Syed
Cardiac rhythm management devices (pacemakers and defibrillators) use an outside source of electricity to stimulate cardiac tissue in diverse ways to produce a therapeutic effect. In the case of pacemaker’s extra impulses are delivered to activate the heart while in the case of defibrillators, energy is used to terminate lethal arrhythmias and restore normal sinus rhythm. Advances in technology over time have changed the field of electrophysiology significantly over a relatively brief time. Looking at the way a 12 lead EKG is acquired. It has changed dramatically with the initial large cumbersome tools to the current handheld devices that can give us at least a 6-lead strip (Figure 1).
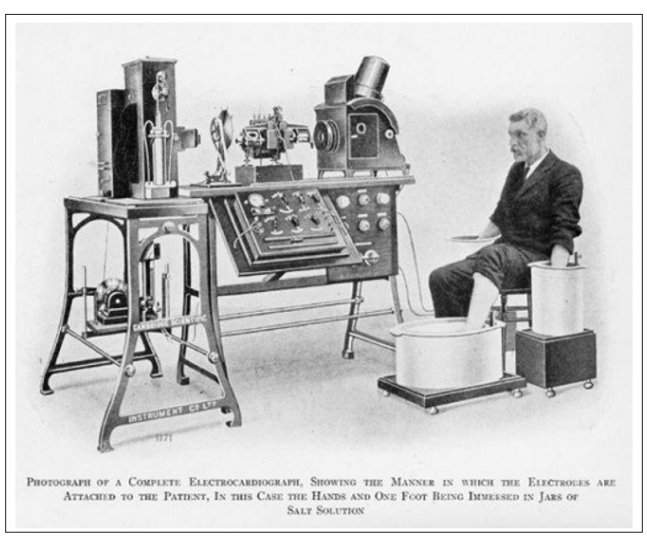
Figure 1a: Early EKG Machine
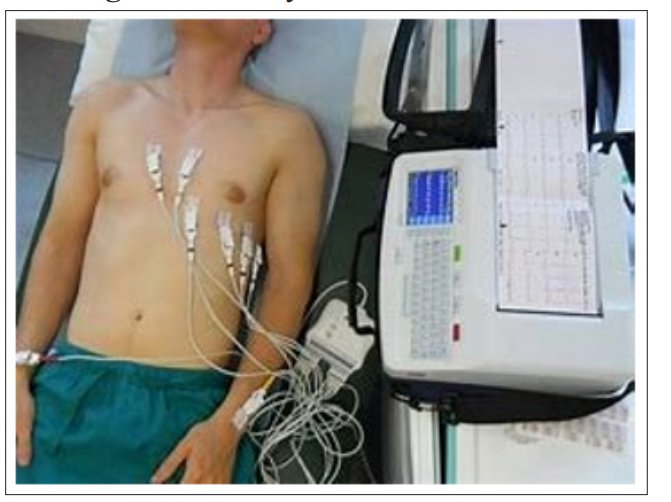
Figure 1b: Standard EKG Machines
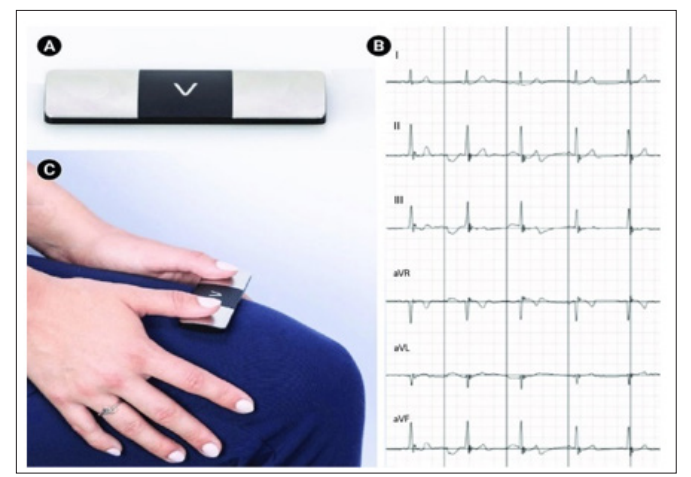
Figure 1c: Portable 6 lead EKG
Initial advances and development of pacemakers was seen in the pediatric population. The researchers faced a lot of criticism. It was a collaboration between surgeons, medical physicians and engineers. The first external pacemakers were reported in 1920’s-1930’s. Mark Lidwell in 1928 used alternating current to try to stimulate the heart. His experiment required a needle to be inserted into the patient’s chest. Albert Hyman in 1932 created a similar device but used a hand cranked motor (artificial pacemaker) to deliver the electrical impulses. Hypothermia experiments are what helped drive the development of pacemakers. 1940’s-1950’s was when we first come across documented pacemakers. These were all external devices driven by vacuum tubes and the impulses travelled thru the electrode which had to be introduce into the heart.
The pioneer for pacing is Dr. Zoll. In 1950, he did experiments to try to stimulate stand still hearts to resuscitate them using external current and connecting it to the patient thru electrode [1]. The first design compromised an electrocardiograph to monitor the rhythm, pulse generator to stimulate the heart and metal electrodes strapped to the patient’s chest. In essence it was a large tabletop pacemaker. required connection to electricity. Patients suffered from skin burns and were unable to tolerate for prolong periods of time due to painful contractions. The first design required manual activation and deactivation and overtime research wsdoen to try to develop battery operated devices.
In 1956, Dr. Leatham duplicated Dr. Zoll’s work and developed the concept of automatic onset and termination of stimulations to pace the heart. By1957, only transcutaneous pacemakers were available, but even those required steel surgical sutures. In 1958, transvenous pacemakers were introduced. The first patient had the electrode placed in the myocardium, but it exited the chest wall to be connected to the outside generator. There were case reports of rechargeable batteries pacemakers as well, however those did not last long at all. Following which, there are reports of a 12 V battery being used. The lead was implanted via a left thoracotomy and brought out the chest wall. The first bipolar electrode was implanted in 1959 and a first completely transvenous system was implanted in 1962. The initaldevices had limited or no programing capabilities. In 1957, external dials were used to set programming options. 1963-63, the pacemakers had needle driven potentiometer. 1961-1973 is when magnetic switches were used. Finally, radiofrequency energy was introduced to set programming options for these devices since 1973 (Figure 2).
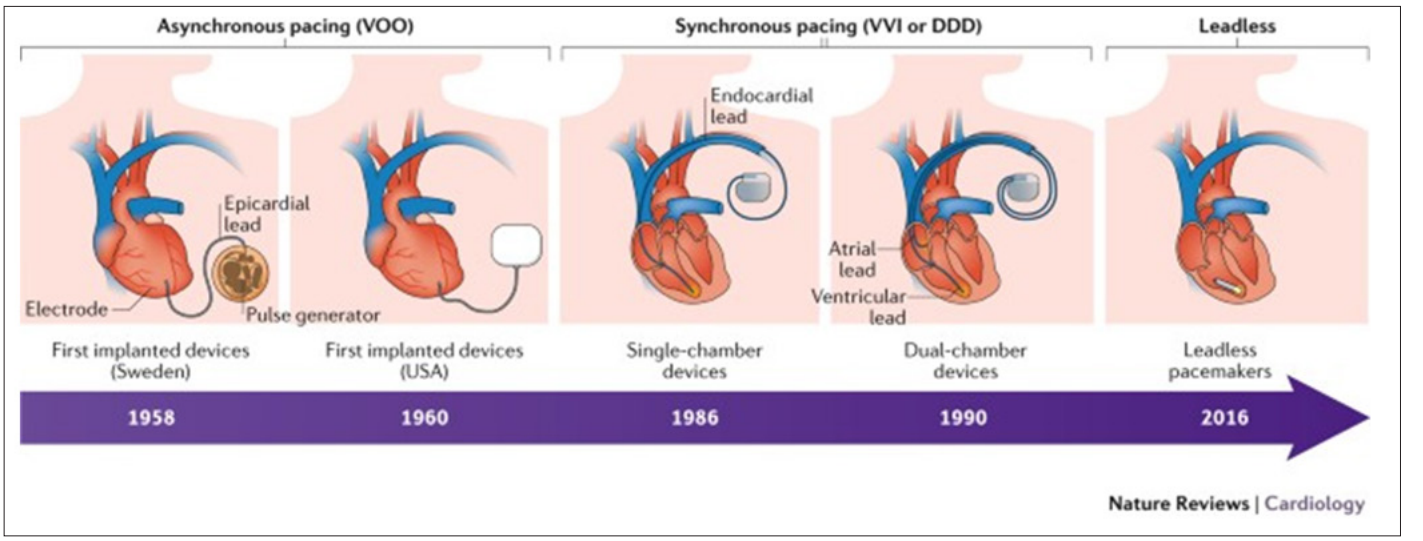
Figure 2: Timeline of Pacemakers
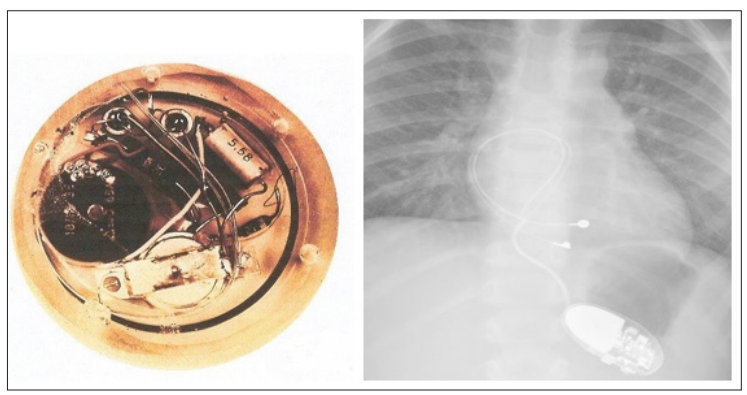
Figure 3a: First Implantable Pacemaker
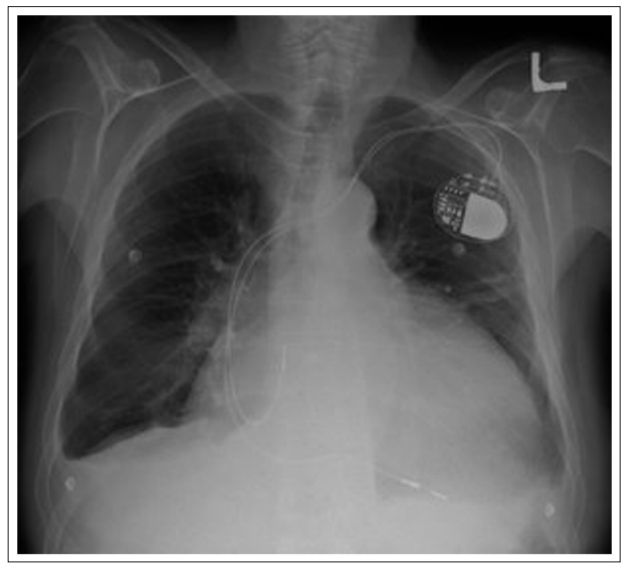
Figure 3b: Transvenous Pacemaker
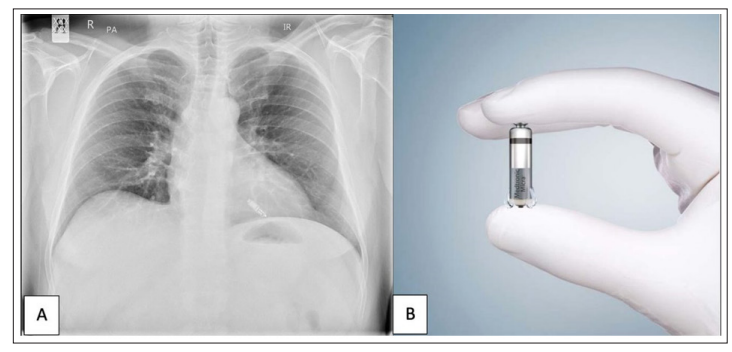
Figure 3c: Leadless Pacemaker
The credit for implantable cardiac defibrillator (ICD) implantation goes to Dr Mirowski Dr. Mower. In 1973 they published their first paper on the success of defibrillation using transvenous catheters in the operating room during bypass surgery. They were however met with a lot of skepticism. The first trials on humans with implantable ICD were in 1980. Patients had to survive two cardiac arrests to be enrolled, and the procedure was done with thoracotomy and epicardial patches were used for defibrillation. From 1984- 1993 was the time that saw the development of transvenous endocardial leads for defibrillation. Initial ICD’s were implanted for secondary prevention only. 3 secondary prevention trials were performed demonstrating benefit from device implant. Enrollment in these trials was poor due to a multitude of reasons including enrollment criteria and type of procedure. Subsequently primary prevention trials were done. The development of transvenous leads and better implant procedures lead to easier enrollment. 8 major primary prevention trials were done all demonstrating a significant mortality benefit with ICD implants. ICD timeline development progressed rapidly with these clinical trials and industry support (Figure 4) The generators also underwent a lot of clinical research over time leading to smaller more compact chest devices compared to larger abdominal implants.
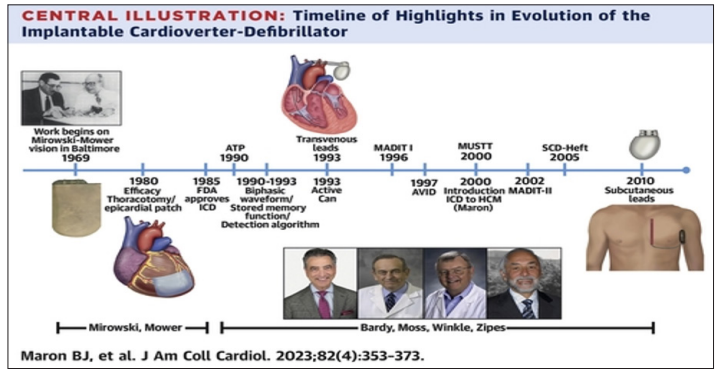
Figure 4: Timeline of ICD Development
Hypertrophic Obstructive Cardiomyopathy (HOCM) was the first go of the inherited channelopathies that was researched by trials and received an ICD which demonstrated benefit. The first trial that was done and dmeotarated a benefit from ICD implant was done in 2000. The recommendations for device implant in these patients are based on primary or secondary prevention. Secondary prevention guidelines are like CHF patients. The primary prevention recommendations for this patient population are somewhat different. The criteria for device implant include: Unexplained syncope, family history of SCD, LV wall thickness > 3mm, NSVT and late gadolinium enhancement. The patients tend to be younger and the long-term risk of an implanted device are different in this patient population compared to CAD/CHF patients. HOCM is usually not associated with the development of end stage heart failure. The issue with inappropriate therapy due to AF or oversensing of T waves has decreased over time due to mainly programming options [2-5].
Arrhythmogenic Right Ventricular Dysplasia (ARVD) is a familial cardiomyopathy with an autosomal dominance inheritance pattern. The abnormal is due to gene variations of the desmosomal proteins. Fibrofatty replacement of the myocardium is noted. There is typically RV involvement as well. There is robust benefit for ICD implant in this patient population, however all the data is from registries and observational studies but no prospective randomized clinical trials [6].
Long QT syndrome patients are at increased risk of ventricular arrhythmias as well. These patients have no structural abnormality but have a repolarization abnormality evident on the 12 lead EKG. Multiple mutations have been documented, however 80% are due to KCNQ1 (Long QT1), KCNH2 (Long QT2) and SCN5A (Long QT3). ICD implant is usually not the first line treatment in these patients. Beta blockers (especially in LQT1) are the first line treatment for these patients. Indication for ICD implant is usually syncope, especially while on beta blockers and QT duration > 500.
Brugada syndrome is another inherited arrhythmia. It has a autosomal dominant inheritance pattern and is seen more frequently in male patient population. The most common mutation is seen in the SCN5A gene. This is another channelopathy that has registry data but no randomized clinical trials demonstrating ICD benefit for secondary prevention. ICD’s are not recommended for EKG findings and/or family history of SCD [7-10].
Current guideline for ICD implant (generally)
