Author(s): Ali Amanati
Persistent marrow aplasia is a rare complication with poor prognosis after intensive chemotherapy for acute myeloid leukemia. We present a 14-year-old boy with acute myeloid leukemia (AML), was complicated by chemotherapy induced persistent aplasia and he was expired because of prolonged neutropenia and pulmonary Aspergilosis. In this review we explain causes of persistent post chemotherapy persistent aplasia and prevention of this phenomenon during treatment with consideration of minimal residual disease (MRD) and response to question about chemotherapy titration dose.
Our patient was a 14 years old- boy with non-M3 acute myeloid leukemia (AML) diagnosis characterized by a normal cytogenetic profile. The patient had an Eastern Cooperative Oncology Group performance no limiting factors for induction chemotherapy with cytarabine, etoposide and daunorubicin according to the EORTC status score of 0 and good cardiorespiratory function was treated by 5 courses of MRC12 chemotherapy protocol.
The patient started chemotherapy 5 days after hospitalization. According to the IFI prophylaxis protocol at Amir oncology hospital, the patient received oral posaconazole 200 mg thrice daily. Numerous adverse events occurred during the period of aplasia, among them grade 4 oropharyngeal mucositis that required parenteral nutrition, and grade 4 diarrhea with severe electrolyte imbalance, which led to the conclusion that posaconazole was an unsuitable treatment option for the patient.
On day 13 of chemotherapy in 3 th chemotherapy course the patient had respiratory complaints, which consisted of productive cough and pleural pain that was more intense in the right hemithorax. He had a 7-day history of fever and received empirically intravenous Meropenem 1 g every 6 h and vancomycin 500 mg q6h as a continuous infusion.
A chest radiogram revealed an infiltrate in the lower half of the right hemithorax with homolateral effacement of the costophrenic angle. A high resolution computed tomography (CT) scan disclosed a pleural effusion of moderate volume on the right side, with consolidation of the lower right lobe and additional smaller foci of consolidation in the middle lobe and lingula. Also was reported a lesion 2.5 cm suspecius to pulmonary abscess. The CT scan was repeated some days later to confirm the previous findings (Figure. 1). A bronchoscopy was performed but the bronchoalveolar lavage did not reveal a pathogenic agent. Blood cultures as well polymerase chain reaction tests for Aspergillus and Candida spp were negative.
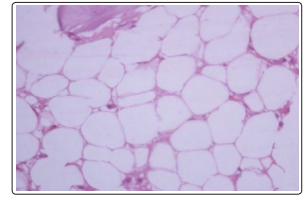
Figure 1: Shows hematopoietic precursors are markedly suppressed on 14 th day after chemotherapy
A galactomannan test could not be exist this time in our center. Given these results and the patient’s clinical deterioration, the patient started voriconazole at a dosage of 4 mg/kg intravenously every 12 h, preceded by a loading dose of 6 mg/kg, on day 18 of chemotherapy (aplasia day 17). On the next day, the patient was admitted to the intensive care unit in septic shock, which required orotracheal intubation, sedation and hemodynamic support. The CT scan was repeated and showed a persistent necrotizing pneumonia with an increase in the cavitation and a collection ofliquid, as well as infradiaphragmatic extension of the abscesslike lesion.
Finally the patient underwent a lobectomy of the lower lobe of the right lung; histologic examination of a specimen showed areas of unequivocal fungal disease morphologically compatible with an infection by Aspergillus spp. (Figure. 2). This finding was a reason to switch her antifungal therapy to liposomal amphotericin B, 5 mg/kg per day intravenously. Under this regimen the clinical symptoms improved and after 5 days of treatment with liposomal amphotericin B the patient could be transferred back to the Hematology/Oncology Unit. Two weeks after the start of liposomal amphotericin B, a CT scan revealed moderate pleural effusion of the right hemithorax and a number of gas bubble-like structures.
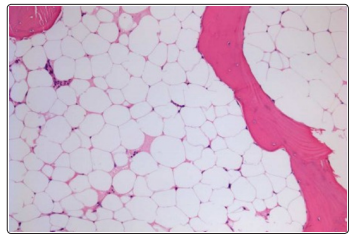
Figure 2: Shows hematopoietic precursors are markedly suppressed on 28 th day after chemotherapy
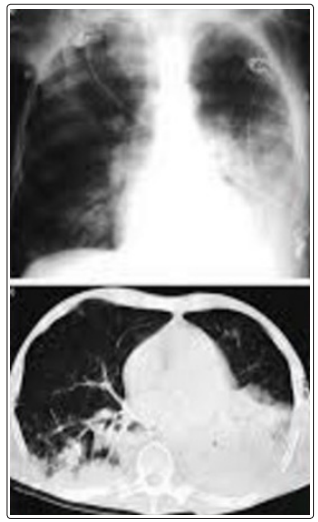
Figure 3: Chest computed tomography image showing left upper lobe cavitary lesion consistent with invasive pulmonary aspergillosis (IPA)
Given the patient’s age and clinical recovery, and considering that her fungal infection seemed under control and her leukemia in complete remission, as assessed on days 30 and 60 postchemotherapy,but leukocyte count and neutrophil count weren,t elevation. Bone marrow aspiration biopsy was performed.It revealed severe aplastic bone marrow after 2 months of Intravenous liposomal amphotericin B, at a low dose of1 mg/kg per day was given as secondary IFI prophylaxis, but during the subsequent aplastic period the respiratory symptoms reappeared and a CT scan showed an increase of the pre-existing pleural effusion.
A chest drain was placed and the dose of liposomal amphotericin B was adjusted to the previous level of 5 mg/kg per day, which resulted in a resolution of the respiratory symptoms. After 90 days in hospital, the he dead due to iatrogenic persistent chemotherapy induced marrow aplasia and pulmonary invasive Aspergilosis following this adverse event.
In first course he involved by acute Graft Versus Host Disease(GVHD) and severe upper and lower Gastrointestinal bleeding(GIB).Chemotherapy after first course was titrated 10- 15 % drugs dose empirically. Despite of drug dose adjustment he involved by frequent prolonged neutropenia periods and ultimately persistent marrow aplasia without any evidence of relapse.
Post chemotherapy Persistent aplasia is a rare complication after intensive chemotherapy for acute myeloid leukemia, leading to a high rate of morbidity and mortality. This problem was rarely reported in the literature [1]. The intensive chemotherapy in sensitive patients such as elderly patients or under nutrition situation might lead to an increased risk of persistent aplasia after chemotherapy [2]. In these sensitive high risk AML patients for preventing aplasia and finding complete remission may be need to dose adjustment during induction chemotherapy base on minimal residual disease(MRD) before bone marrow transplant [3].
Although the incidence and pathogenesis of chemotherapyinduced persistent aplasia is still unknown, or may be rare, but this condition need to screen for sensitive patients and high risk condition because of prevention of fatal events [4]. In this clinical setting critical lethal period between permanent chemotherapyinduced aplasia and hematopoietic stem cell transplant (HSCT) associate with life-threatening infections such as pulmonary aspergillosis or mucormycosis infection and cytomegalovirus (CMV) pneumonitis. Therefore find a correlation between Minimal Residual Disease (MRD) and predictor factor for chemotherapy induced aplasia is inserted the patient in equilibrium between disease remission and prevention of iatrogenic marrow aplasia.
The data were collected from Pubmed, MEDLINE, EMBASE, google scholar and CINAHL and relevant websites since 1995 until Agust 2015. English language papers were included and expert opinions or seminars’ information such as ASH annual meetings were also collected. Inclusion criteria for selected articles were:1-publication date between 1995 till 2015. 2- There would be enough similarities between the studies selected to make combining them reasonable. Exclusion criteria were: 1- Conference proceedings. 2- Articles published in a language other than English. 3- Systematic reviews, if they were meeting abstracts not subsequently published in peer-reviewed journals, editorials, commentaries, letters, news articles, case reports, or narrative reviews. All studies have been screened for children aged 0-18 years or weak elderly adult patients
Review articles included 19 studies about “acute myeloid leukemia, chemotherapy induced persistent marrow aplasia, minimal residual disease.” in children, although observed heterogeneity rate was high (I2=82%). Heterogeneity was evident in both age groups (adult and pediatric) and could not be explained by the status of randomization or the type, duration, or extent of the intervention. Two review authors independently extracted the data and assessed the risk of bias of included studies. The review also included some additional and related studies found on the following keywords: “acute myeloid leukemia, chemotherapy induced persistent marrow aplasia, minimal residual disease”. The research team developed a study protocol to address research questions for the pediatric patient populations with acute leukemia.
A total of 31 publications (31 and 246 exclude) during 1995- 2015, met eligibility criteria and form the evidentiary basis for this review.
Date & place: Iran-Shiraz-Amir oncology hospital: Dec-Feb 2015. We found some risk factors for intensifying chemotherapy induced marrow aplasia in the literature and rule out these factors for our patient.
It is an uncommon life- threatening hematologic disorder. It is a disease with severe inflammation caused by uncontrolled proliferation of activated morphologically benign lymphocytes and macrophages and increase high amounts of inflammatory cytokines. It is classified as one of the subgroups of histiocytosis class 2 [5]. In hematologic cancers, HLH is classically associated with specific cell phenotype, such as T-cell or NK/T-cell lymphoma and intravascular large B-cell lymphoma, or caused by bacterial, viral or fungal infections and is, thus, frequently described as malignancy- or infection-associated hemophagocytic syndrome. In acute myeloid leukemia (AML) patients, HLH has been rarely described in case-reports. AML patients may be prone to develop HLH due to their disease- or treatment-related impaired immune response and their high susceptibility to severe infections, which trigger prepheral factors [6].
It is a rare complication of blood transfusion, in which the donor T lymphocytes mount an immune response against the recipient’s lymphoid tissue. In this situations where the recipient is immunocompromised due to chemotherapy, the recipient’s immune system is not able to destroy the donor lymphocytes. This can result in graft-versus-host disease. Graft-versus-host disease is usually observed after allogeneic BMT but is occasionally recognized after transfusion or solid organ transplantation.
Transfusion associated (TA)-GVHD usually occurs in the immunosuppressed recipient (BMT recipients), but new reports also involve more nearly immunocompetent recipients. Since there are no pathognomonic manifestations of GVHD, this syndrome isn,t sometimes easy to distinguish from viral infections or drug eruptions. TA-GVHD is usually severe, and, unlike the condition after allogeneic BMT, it frequently results in pancytopenia secondary to marrow aplasia. The most of reported cases of TA-GVHD have not responded to immunosuppressive therapies and have been fatal.
Reports of TA-GVHD after transfusion of cellular blood products from homozygous blood donors to heterozygous non-blood relatives suggest that indications for gamma irradiation may need to be broadened. The risk of transfusion of blood from HLA homozygous donors to unrelated HLA heterozygous patients is 1 in 874 among Japanes patients and may be as high as 1 in 7,174 in the United States. Finally, reports confirm that all HLA-matched cellular components should be irradiated [7].
Hepatitis C virus infection in patients with resistant form of the aplastic anemia is one of the principal causes of the bone marrow aplasia. Herpes simplex virus (HSV) infection also is an indirect cause, and might substantially aggravate BM aplasia [8]. We didn’t find any evidence of PVB19 as cause of permanent chemotherapyinduced aplasia and pancytopenia.
In one study on 7 patients with acute myeloid leukemia, in Russia, had developed deep bone marrow aplasia (BMA) due to exposure to chemotherapy, were analyzed. In this study compared in all the patients the values of peripheral blood and bone marrow (BM) aspiration samples and the results of blood tests using the polymerase chain reaction at different acute leukemia development stages with the results of an immunohistochemical study using the markers of viruses of hepatitis C and B, a herpes group (EBV, CMV, HSV-1, HSV-2) and parvovirus B19. Hepatitis C was detected in 6 of the 7 patients with prolonged BMA; 3 of these 6 patients showed infection with hepatitis B. Six of the 7 patients were found to have concomitant BM lesion with various herpes group viruses. Two patients had a resistant form of AL [8].
Acute myeloid leukemia in 3-5 % of Fanconi anemia patients is first hematologic manifestation.Fanconi anemia is characterized by congenital abnormalities, fail hemopoiesis and high risk of developing acute myeloid leukemia (AML) and certain solid tumors. Its total prevalence is 1 to 5 patients/million with a carrier frequency of 1:200 to 300 in most populations. These estimations are based on the incidence of affected individuals before the full spectrum of the FA phenotype was recognized [9].
Mutations in FANCD1/BRCA2, has a cumulative incidence of cancer of 97% by age 7 years; the cancers are AML, brain tumors, and Wilms tumor; several patients have multiple events. Patients with the other genotypes (FANCA through FANCQ) have cumulative risks of more than 50% of marrow failure, 20% of AML, and 30% of solid tumors (usually head and neck or gynecologic squamous cell carcinoma), by age 40, and they too are at risk of multiple adverse events) [10].
FLT3 ligand is a hematopoietic four helical bundle cytokine. It acts as cell-surface receptor for the cytokine FLT3LG and regulates differentiation, proliferation and survival of hematopoietic progenitor cells and of dendritic cells. Activation of wild-type FLT3 causes only marginal activation of STAT5A or STAT5B. Mutations that cause constitutive kinase activity promote cell proliferation and resistance to apoptosis via the activation of multiple signaling pathways.
FLT3 in serum inversely correlated with the colony-forming ability of Aplastic Anemia bone marrow precursors. Expression of FLT3 was significantly elevated in mononuclear bone marrow and peripheral blood cells among patients with severe pancytopenia. Flt3 ligand level reflects hematopoietic progenitor cell function in aplastic anemia and chemotherapy-induced bone marrow aplasia [11]. In this part minimal residual disease is second plate of equilibrium between BM remission and marrow reproduction state.
MRD frequency assessments by real time quantitative (RQ-PCR) and multi parameter flowcytometry (MFC) in AML patients are more sensitive methods to evaluation of bone marrow remission compared to morphologic assessment. Although RQ-PCR is in general the most sensitive technique, MFC is applicable in almost all AML patients [12, 13] MRD status will be implemented in clinical decision-making. Sometimes an alternatives for BM MRD include MRD assessment in peripheral blood and blast reduction, frequency of B-lymphocytes precursors and CD34+ myeloid lymphoid ratios are administered [14, 15].
An alternative, probably more specific method to predict clinical outcome is leukemic stem cells Ag( LSC) frequency assessment. Results so far on the clinical importance of LSCs are limited, but very promising, especially since for the first time the correlation between the presence of LSCs after treatment and clinical outcome has been reported. When the value of LSC assessment is confirmed in future retrospective and eventually prospective studies, it may be hypothesized that in the future, not only MRD, but also LSC frequency assessment may be implemented in clinical decision-making. In the field of Minimal Residual Disease (MRD) AML compared with acute lymphoblastic leukemia has limitation. Only half of AML patients have suitable molecular target for MRD monitoring [16, 17].
1. Fusion molecular markers: AML1/ETO and CBFβ/MYH11 PML/RARα, FLT3-ITD
2. P-glycoprotein (a multidrug resistance modulator), Bcl-2 and Bcl-XL, PRAME mRNA Levels.
3. Multi-parametric flow cytometer (MFC) factors such as: CD25 or chain of the IL-2, CD34 and CD117, CD117+/CD15 expression, CD45 by leukemic myoblasts.
4. Nucleophosmin1 (NPM1) mutation provides a sensitive marker for MRD detection by RT-QPCR in about 30% of AML cases.
5. Real PML- & RARA-time quantitative PCR to detect PML
6. RARA fusion transcripts generated by the t(15;17) in APL ort(8,21) detected by PCR.
7. Leukemic stem cells antigen (LSCs). [18-25].
The reasons for the persistent aplasia in AML patient are mainly unknown but first evaluation in this situation is leukemia relapse exclusion by bone marrow histology. A severely defective bone marrow stroma was rule out by the fast regeneration of hematopoiesis after temporary marrow aplasia induced by chemotherapy. Some risk factors might have been significant effect on post chemotherapy persistent aplasia which include: Immune events such as hemophagocytic syndrome, transfusion associated GVHD, viral infections such as Hepatitis C virus and Herpes group except PVB19, occult Fanconi Anemia, FLT3 ligand serum level. Patients with acute myeloid leukemia may be prone to develop hemophagocytic lymphohistiocytosis because of an impaired immune response due to receive high dose chemotherapy and destroy primary immune system structure. One of the main presentation of this syndrome is pancytopenia induced cytokine release and marrow aplasia [6,7,8].
Transfusion associated GVHD presenting as bone marrow failure due to non-irradiate transfusion. It generally does not respond to immunosuppressive therapy, and it is almost always fatal, but our patient lived after this undesirable condition. Given the grave prognosis and unrelenting clinical course of this disease, efforts must be placed on prevention. Since adequate gamma irradiation of cellular blood components before transfusion can entirely prevent GVHD, it is critical to recognize the patients at risk so that irradiated cellular blood components can be administered [26].
Hepatitis C virus infection in patients and the resistant form of the disease were the principal causes of the development of BM aplasia dependent cytostatic drug exposure [27]. Affliction of abundant bone marrow cells with herpes group viruses was not a direct cause, but might substantially aggravate BM aplasia but in Hepatitis-associated aplastic anemia is a distinct type of aplastic anemia. The cause of the hepatitis is unknown, but it does not appear to be due to any of the known hepatitis viruses [28].
Although we didn’t find any evidence about role of Parvovirus B19 in infection should be included in the differential diagnosis of unexplained severe chronic anemia and thrombocytopenia in the immunocompromised, including HIV-positive and organ transplant patients. Elevated plasma FLT3 level in patients who previously received chemotherapy is a predictive measure of the stage of recovery of the bone marrow compartment [29].
Awareness about bone marrow activity should permit that induction chemotherapy be enough aggressive and prevent over treatment myelosuppression toxicity and finally prevention from iatrogenic permanent therapy related aplastic anemia. In AML patients, morphologic assessment is performed to evaluate chemotherapy response and to define remission status. By definition, patients are in complete remission (CR) when less than 5% blast cells are present in the bone marrow (BM) concurrent with evidence of normal erythropoiesis granulopoiesis and megakaryopoiesis.
In addition, neutrophils and platelets in peripheral blood should be at least 1.0 x 109/l and 100 x 109/l, respectively. Since about 50% of patients in CR will eventually experience a relapse, for this reason more precise assessment of the quality of CR is necessary. Quantitative MRD frequency assessment could give important prognostic information after chemotherapy treatment. Two highly sensitive methods for MRD detection in leukemia are multi-parameter flow cytometry (MFC) and real-time quantitative polymerase chain reaction (RQ-PCR) [30].
Theoretically, we suggest to treat every patient with AML individually according to risk allocation, which must include MRD levels and FLT3 serum level. This approach would intensify treatment or switch chemotherapy to an MRD-targeting strategy in MRDLOW/- patients. MRD+ patients and reduce treatment intensity in Often for AML patients allogeneic bone marrow transplantation is done after remission documented by MRDLOW/- and patients should be treated by sub-myeloablative conditioning chemotherapy if who beginning of permanent marrow aplasia [31].
In pediatric AML, MRD should be added to conventional risk stratifications such as acute promyelocytic leukemia (APL) patients. In conclusion, we recommend following points for better management of AML cases:
1. Monitoring of serial MRD during chemotherapy period
2. Monitoring of serial FLT3 ligand level during chemotherapy period
3. Application of irradiate blood products in AML patients
4. Evaluation of patient for viral infections, chromosomal breakage in start of induction chemotherapy
5. Dose titration of chemotherapeutic agents for prevention of post chemotherapy aplasia and its complications base on MRD & FLT3 equilibrium
6. In conclusion, if iatrogenic marrow aplasia happen despite of administration of mentioned precautions then nonmyeloablative transplant conditioning regimen was considered for preventing fatal events.
