Author(s):
Dysregulated glucose metabolism promote inflammation in monocytes and macrophages from patients with atherosclerotic coronary artery disease. Men with metabolic syndrome are at increased risk for sudden cardiac death, and the incident sudden death is not explained by obesity or traditional cardiovascular risk factors (Kurl, 2016), but the ingested nutritional iron. Individuals with increased abdominal adiposity exhibit an increased risk of heart failure, in spite of there are oweweigtht or not (Cavalera, 2014), because the insulin resistance contribute to increased myocardial fibrosis in the absence of hypertension (Quilliot, 2005). Iron overload, and plasma viscosity contributes to cardiovascular risk in the general population, particularly in men (Van der A, 2005; Junker, 1998). Iron influences glucose metabolism, even in the absence of significant iron overload, and its reduction may alleviate coronary heart disease and reduced or prevent their complications: High stored or free iron levels (measured by serum ferritin or catalytic iron concentrations) elevate risk for development of coronary atherosclerosis, because labile iron accelerates endothelial dysfunction and originates oxidative injury that promotes systemic and vascular inflammation, phrothrombotic conditions and insulin resistance (Williams, 2002). High serum ferritin is strongly and independently associated with acute myocardial infarction and constitutes a novel risk factor in
acute sudden event (Holay, 2012). Iron plays a direct and causal role in diabetes pathogenesis mediated both by β-cell failure and insulin resistance (Simcox, 2013). In the general population, body iron stores are positively associated with the development of glucose intolerance, type 2 diabetes and gestational diabetes. In this way, blood donation significant drops in the incidence of cardiovascular events, as well as in procedures such as percutaneous transluminal coronary angioplasty and coronary artery bypass grafting (Holsworth, 2013): frequent blood donations decreased iron stores in healthy volunteers, improving insulin sensitivity and hemodynamic parameters. Iron and oxygen-derived free radicals are important in the pathogenesis of postischemic reperfusion injury and contributes substantially to endothelial dysfunction in acute coronary syndromes (Chekanov, 2002; Duffy, 2001) , and a high iron diet potentially increase ischemic damage induced by transient ischemia and early reperfusion (García-Yébenes, 2012) in animals and humans. Iron, hyperinsulinemia, and hyperglycemia act in concert to up regulate free-radical reactions (Facchini, 2000) and this metal excess accelerated the development of atherosclerosis and its accumulation may promotes illness, particularly, ischemic cardiovascular diseases. Insulin resistance in macrophages promotes formation of a necrotic core in atherosclerotic plaques by enhancing macrophage
apoptosis, and exposure it to circulating blood in the event of plaque rupture can precipitate thrombosis, leading to unstable angina pectoris, myocardial infarction and sudden death (Rask.Madsen, 2012, rev). In humans phlebotomy slows progression of peripheral vascular disease and blood donation lowers significantly the risk of myocardial infarction (Salonen, 1998), particularly in insulin-resistant subjects. On the contrary, exogenous iron into healthy individuals provoked endothelial dysfunction accompanied by increased generation of superoxide radical in whole blood (Vinchi, 2014, rev). A causal relationship between pre-diabetes and cardiovascular disease exist (Ford, 2010, rev). Humans lack effective mechanisms to excrete excess iron, and excessive dietary iron uptake cause iron deposition in heart, and pancreas (Kulaksis, 2008) leading to sudden death and occult diabetes mellitus. Iron play an underappreciated role in the development of insulin resistance and insulin resistance-induced heart failure. In a chronic and acute way, Insulin resistance is an early and major factor in the development of heart failure and acute iron induced insulin resistance in cardiomyocytes (Sung, 2019).
1- Introduction
2- Catalytic Iron; Irreversible Oxidizer
3- Excess Hemoglobin and Cardiocerebrovascular Pathology
4- Free Iron as a promoter of Ischemic Heart Disease
5- Excess Ferritin and pathogenesis of Endothelial Dysfunction
6- Hyperinsulinemia as a Sudden Death Promoter. The determining role of Iron
7- Iron accumulated in excess and pathogenesis of Diabetes: High hemoglobin versus High Ferritin
8- Iron and atherosclerosis. The evidence
9- Blood Donation. Cardiovascular Pathology Protector
The development of emergency medicine has highlighted something forgotten until now: physiological and pathophysiological patterns are a consequence of underlying metabolic abnormalities of greater importance: thus, cellular metabolic insufficiency is the basis of acute cardiorespiratory pathologies (Siegel, 1983, rev). Insulin is a vascular hormone that regulates vascular tone and reactivity (Novo, 2016, rev), modulates the degree of arterial stiffness; (Westerbacka, 2001), the structure, function and endothelial senescence (Matsui-Hirai, 2011), microvascular, arteriolar, arterial, aortic, hemodynamic, metabolism and consumption of myocardial oxygen (Von Bibra, 2010), physiopathologically regulates coronary flow (Sundell, 2003, rev); and that it establishes its vasoregulatory, autonomic and capillary recruitment effects (anti-inflammatory included) faster than its classic metabolic effects (excellent rev, in Liu, 2019 rev; Novo, 2016, rev; Kim, 2008, rev; Matsui-Hirai, 2011, rev; King, 2016, rev; Westerbacka, 2001); in such a way that Insulin Resistance can be a causal factor - in non-diabetics - of the extensive ischemic cardiovascular pathology, but, in addition, it is associated with myocardial and vascular subclinical alterations in subjects without significant coronary damage, by increasing arterial stiffness (Novo, 2016, Liu,2019, rev). It has been recently shown that catalytic iron increases vascular, adipose and hepatic resistance to insulin (Jahng, 2019). Chronic iron overloads in rodents progressively damage the myocardium causing intesticial and perivascular fibrosis, with cardiomyocyte apoptosis; and myocardial iron levels are similar to those of patients with cardiomyopathy and heart failure (Oudit, 2004). Therefore, it should not be surprising that, excess iron, the metal with the highest oxidation, has been suggested with clinical evidence, be it a key event in the generation of acute thrombosis, as a result of a nocturnal increase in Insulin resistance (and endothelial dysfunction), and leading to a heart attack (Opie, 1995, rev). In addition, and as recently demonstrated, a sudden death - eg, by coronary arterial vasospasm can be prevented - even in nondiabetics - by improving Insulin Sensitivity with insulin and diet medication (Kang. 2018). It is categorically established that the athero-thrombotic process, both in each of its components and in its syndromic presentation, is exacerbated or initiated because of Insulin Resistance, promoted, in turn, by an overnutrition (Williams, 2016, rev), where excess daily iron plays a key role (Fernández-Real, 2002). There is strong epidemiological evidence that iron is a key factor in the development of the atherosclerotic process (de Valk, 1999); on the contrary, acute insulin in the presence of a good tissue sensitivity to it has been shown to have anti-atherosclerotic capacity (Hsueh, 1999, Dandona, 2004, Low Wang, 2003) (which evidently cancels in the insulin-resistant common individual and/or sedentary). It has been known for two decades that, physiologically, insulin optimizes tissue capillary recruitment (Coggins, 2001). A pathophysiological fact repeatedly proven, but forgotten in Emergency Medicine: the excess of upper body fat as a reflection of a chronic excess of Insulin - hyperinsulinemia / insulin resistance- is a clear cardiovascular risk factor in non-diabetic population (Haffner, 1988). Thus, this android distribution of body fat is reflected more accurately if one considers the circumference of the thigh in relation to the supine sagittal abdominal diameter (Kahn, 1996).The epidemiological association between Insulin Resistance, coronary atherosclerosis and arterial hypertension is much more common than is suspected, in view of experimental and clinical evidence that microvascular angina is also characterized by resistance insulin and a coexisting or resulting endothelial dysfunction (Goodfellow, 1996). And the metabolism of iron altered, induced or increased by the current diet does not escape it. It is shown that endothelial dysfunction precedes hypertension in models of rapid insulin resistance induced by fructose (on the tenth day), (Katakam, 1998), evidencing that it is only the moderate Insulin Resistance that causes early endothelial dysfunction and precedes the onset of arterial hypertension, and regardless of the presence of obesity (Romanko, 2009). Oxidative processes mediated by free or catalytic iron contribute greatly to myocardial necrosis that occurs during ischemia and reperfusion: this was demonstrated reliably in animals when the chelation (“tissue sequestration”) of metal, was shown to reduce canine infarction in its size and oxidative injury, 24 years ago: early treatment limits myocardial ischemia and reperfusion cell damage (Lesnefsky, 1990, Reddy, 1989). It will be almost three years since it has been proven that the reactive oxygen species -ROS- induce cell death by apoptosis of the myocardial cell (Aikawa, 2002). However, cell, tissue, and especially vascular damage continues to be ignored, which the acute / excessive increase in ROS, generates and perpetuates in the cumulative and progressive damage to the cardiovascular system. Heme iron is a powerful contributor in the formation and progression of atherosclerosis by catalyzing the production of oxygen free radicals - ROS - and promoting the oxidation of LDL cholesterol (Abdalla, 1992): a greater deposit of body iron has long been shown, be associated with a greater severity of vascular lesion in animals and humans. Although isolated iron is not sufficient to induce per se the new formation of vascular lesions, the different preclinical animal models have shown their harmful role in the promotion and aggravation of vascular disease (Ishizaka, 2002), and the inflammatory -oxidant role of iron- is even greater in combination with inflammatory fats, which promote very rapidly cardiac instability in non-diabetic subjects (Marfella, 2001); It is the combination of both, but especially of iron loads, which greatly aggravates insulin resistance, causing mitochondrial dysfunction and reduction of HDL-cholesterol (Gao, 2009, Choi, 2013), in addition to raising blood sugar. And, we all know, today that Insulin Resistance is the promoter or aggravating event of ischemic heart disease (Reaven, 2011; Reaven, 2012). It is repeatedly proven that insulin resistance (IR) is in itself the most important factor for the acquisition of coronary artery disease; optimal hormonal sensitivity is decisive for the maintenance of a complete structure and adequate function. Endothelial (Sowers, 1993, Yudkin, 1998, Scherrer, 1997, DeFronzo, 1991, Haffner, 1999; Mather, 2001, rev). Iron plays a pathogenic role in reperfusion injury, abnormal iron accumulation diseases (hemochromatosis, beta-thalassemia); in anthracycline cardiotoxicity; and, particularly in coronary atherosclerosis: all this is checked when iron removal (by phlebotomy or chelation) proves to be a very effective treatment for the reduction of ironinduced cardiotoxicity (Horwitz, 1999). In this regard, the limitation in dietary iron can be decisive for disease improvement. Thus, many researchers continue to report that chelation iron capture is an effective treatment in neurodegenerative diseases, especially in Parkinson’s E., Alzheimer, Huntington and Friedreich’s Ataxia (Richardson, 2004). Cardiac damage caused by chronic iron overload is the leading cause of death in subjects with thalassemia, but the really interesting thing is that irondependent cardiomyopathy is completely reversible with iron capture and chelation, dramatically increasing survival in children. Patients (Kolnagou, 2008). If the most frequent cardiac complication in beta-thalassemia, and cause of death, is Atrial Fibrillation, it is because of iron overload (Nomani, 2018). In general, we must emphasize that fibrillation is increasing in an epidemic way in direct relation to the altered physiology and distribution of adipose tissue (Pabon, 2018, rev).And here the excess iron is involved, which causes Adulin Resistance Adipose (Ma, 2017) and greater intrapericardial inflammation (Pabon,2018, rev) and endothelial, in addition to generating glucose dysmetabolism (Moreno-Navarrete, 2017). In addition, it continues to show that iron accumulated as Ferritin is a powerful inducer of ICAM-1 intercellular adhesion molecules and proinflammatory genes dependent on NfKB factor (Rudell, 2009) at the liver level. Ventricular dysfunction - from moderate to severe - is associated in animals (Oudit, 2004) and humans with high iron body deposits, which have been shown to compromise the patient’s survival in only one year: in addition to the marked symptoms, survival improves dramatically with iron removals by phlebotomy (Horwitz, 1999, rev). Definitely, in subjects with high concentrations of free iron (not bound to transferrin), the metal accelerates the progression of atherosclerosis (Marx, 2008). Iron overloads cause diastolic and systolic dysfunction and promote bradycardia and arrhythmogenesis in humans (Oudit, 2004, SiriAngkul, 2018, rev). A cumulative clinical evidence is showing that iron directly and indirectly affects the action of insulin and glucose metabolism (Ford, 1999; Fernández-Real, 2002; Tuomainen, 1997, Jiang, 2004), clinical evidence that manifests as diabetes and ferrotoxic heart disease in disorders caused by iron overloads, with a relative exception. Hemochromatosis (see below). Regarding the potential endothelial damage that iron potentially exerts, 2 decades ago it was shown that Dexferroxamine, a classic iron chelator, completely inhibits iron-dependent irreversible cytotoxic damage (Thomas, 1993). It is long proven that high levels of iron (and copper) are rapidly mobilized against any tissue ischemia, and are direct and powerful causes of cellular and tissue damage (Berenshtein, 2002). The severity of atherosclerosis is markedly influenced by iron overload, and by its marked deficiency (Chau, 2000). In our western reality, intracoronary calcium levels, a well-known early subclinical marker of coronary atherosclerosis would increase according to serum ferritin levels in non-diabetics; and independently of preexisting cardiovascular disease, and other risk factors (You, 2005, Sung, 2012). In an interesting case-control study in healthy middleaged men and women, serum ferritin was significantly associated with carotid atherosclerosis plaques, and in subjects free of subclinical inflammation (measured by alpha-1 acidic glycoprotein) (Ahluwalia, 2010). It is known that abdominal and / or higher obesity (independent of total weight) causes greater adipokinedependent systemic inflammation than global obesity (Wisse, 2004). The subjects with the highest fasting insulin levels among subjects with abdominal obesity of Hindu or Pakistani origin (living in Europe) are those with the highest rates of premature myocardial infarction and / or sudden death (Kooner, 1998, Iqbal, 2013). Insulin resistance is a determining factor in the pathogenesis of dyslipidemia (Garg, 1996), regardless of the presence of obesity. Iron overloads can lead to the development and progression of Diabetes by promoting insulin resistance and reducing insulin production (Swaminathan, 2007, Tuomainen, 1997). In addition, moderate high reserves of body iron are proving to cause Insulin Resistance, Metabolic Syndrome and gestational diabetes (Rajpathak, 2006, Lee, 2004, Bozzini, 2005, Jehn, 2004, Jiang, 2004, Lao, 1997, Lao, 2003, Chen, 2006).
Iron is an essential molecule for cell physiology; but it is, like many vital trace elements, a double-edged sword: its free form promotes the generation of free radicals, and its excess is a promoter of tissue damage, especially at the cardiocerebrovascular level, by causing lipid peroxidation and promoting reperfusion vascular damage (Kiechl , 1997; Day, 2003), oxidation of LDL cholesterol (Abdalla, 1992, Haidari, 2001), severe vascular oxidative damage (Armaganijan, 2003; Xu, 2008), in particular due to endothelial dysfunction (Day, 2003). Directly, as just demonstrated in vivo, iron is able to promote platelet activation (Praticó, 1999). Body iron plays a contributing pathogenic role in the development of coronary atherosclerosis (Ahmed, 2009). And, it is the form of free iron (catalytic iron) that, by participating in oxidation reactions, generates powerful oxygen oxidizing species which promote (or increase) vascular injury (Rajapurkar, 2012). Although, for more than two decades it has been shown that iron chelation is protective of cardiovascular and renal disease, the crucial importance of catalytic (or labile) free iron has only begun to be seriously recognized (Thethi, 2011). For example, after a vascular lesion it is proven that moderate iron loads accelerate the formation of arterial thrombosis, damaging vasorelaxation (Day, 2003), generating endothelial dysfunction. Free iron in charge generates vascular insulin resistance (inflammation) (Fernández-Real, 2005). To date, there is a double line of evidence that demonstrates the pathogenic role of labile iron in human disease: that where it is increased and generates e.g. cardiotoxicity (Thalassemia), and that in which iron chelating agents provide a protective effect, all of which suppose a powerful causal relationship (Thethi, 2011). The hypothesis of iron body status and cardiovascular disease (Sullivan, 1981) has been corroborated by numerous preclinical, and now clinical experiments that prove the pathogenic role of iron in reperfusion cardiac injury (Reddy, 1989, 1990): thus, it is shown that iron-catalyzed reactions play a significant causal role in myocardial hibernation; Its chelation is capable of reducing post-ischemic ventricular dysfunction (Bolli, 1987). So, today it is proven that iron and derived ROS are determinants in the pathophysiology of post-ischemic reperfusion injury (Van der Kraaij, 1989). In addition, in recent very interesting investigations, the prothrombotic power of metal has been confirmed (Day, 2003): iron is a spontaneous fibrin inducer, which explains the known interrelation between thrombotic diseases and the higher concentrations of hemoglobin or free iron (Pretorius, 2012; Lipinsky, 2012-b). In the presence of chronic insulin excesses, as occurs in insulin resistance - in the extreme common - the catalytic iron pool increases, given the physiological effect of the hormone - even in normal amount - to increase the tissue uptake of iron and increase its availability by tissues (Fernández-Real, 2002), as we will see later.
Insulin resistance is the pathophysiological link between cardiovascular disease and abdominal obesity (Reaven, 2011). In all insulin resistance there is greater blood viscosity (Facchini, 1998) and greater thrombotic potentiality (Kernan, 2002).On the contrary, acute doses of rapid insulin prove to be very favorable for cardiovascular physiology - exogenous, anti-inflammatory and anti-atherosclerotic insulin. Endogenous inflammatory and atherosclerotic insulin (King, 2016): thus, it is the direct effect of an acute dose of exogenous insulin that has been shown to provide survival to the myocardial cell regardless of its metabolic effects (Sack, 2003; Jara, 2003, rev; Feng, 2013), This and all myocardial protection against post-traumatic oxidative stress - proven in a recent clinical study - (Feng, 2013) could be substantially impaired with catalytic iron.The very close positive interrelation between plasma viscosity and the severity of coronary artery disease, even after age correction (Junker, 1998), is fully demonstrated. Accordingly, a positive therapeutic effect of the systolic and diastolic rebel hypertension (renal posttransplantation) of erythrocytosis with 500cc phlebotomies that reduced hematocrit below 45% has been demonstrated (Barenbrok, 1993). Thus, elevated ferritin levels emerge as an early predictor of hypertension (see below) (Ryoo, 2015). Ferritinserum emerges as a significant risk factor for acute myocardial infarction (Silvia,2003, Holay, 2012) especially in our male population.The higher the hemoglobin a particular subject possesses, the greater the release of labile iron during an acute pathophysiological event that destroys (releases) hemoglobin (Haase, 2010). That is, in the presence of reactive oxygen species in a subject with abdominal obesity (insulin resistance), labile iron is always released from the Hem molecule (Gutteridge, 1986).Iron is the largest oxidant in vivo in animals and humans (Araujo, 1995). It has been proven in recent studies that dietary iron, especially in its most bioavailable form (Iron Hem) could increase the risk of type 2 diabetes, as well as coronary heart disease and cardiovascular mortality, especially among women and men non-diabetics (Jiang, 2004a; Jiang, 2004b; Rajpathak, 2009; van der, 2005).In very interesting investigations, Pretorious et al, have shown that, in addition to its inducing effect of profibrine-dependent vascular inflammation (Lipinsky, 2013-c), free iron is capable of altering the morphology of red blood cells: thus, likewise that what happens in diabetic subjects, iron in low concentrations (0.03 mM FeCl3) - similar to iron overloads - alters the ultra-structure of the red blood cell membrane (Pretorius, 2013-c), which are trapped in the Fibrin meshes (up to 70% with the addition of iron, as in decompensated diabetics; compared to 3% in physiological conditions). With all this, we can confirm that iron overload induces pro-thrombotic conditions (Day, 2002; Franchini, 2008; Pretorius, 2013-c) and a potential thrombosis (Day, 2003). It has been shown in mice, and currently in humans, that iron-rich diets worsen cerebral ischemic damage and cause their hemorrhagic transformation (García-Yébenes, 2012).On the other hand, preclinical studies have confirmed the ferric inducing power in the fragility of lysosomal membranes (Schwartz, 2002). Recently, the causal role of iron overload in metabolic syndrome has been proven (Gabrielsen, 2012), mediated by adipocyte.
Although there is a greater consensus between high levels of ferritin with coronary heart disease in insulin-resistant subjects - evidence found more than two decades ago (Salonen, 1992; Giles, 1993; Ascherio, 1994); there are some discrepancies between ironHigh dietary as a cause of coronary heart disease (BorchIohsen, 1993). However, this would be due to methodological problems and biased studies, as will be seen later. In a recent report in obese women, there is a direct chorelation between iron and the mobilization of fatty acids in vivo, which suggests that high body deposits of iron increase insulin resistance, increasing lipolysis and systemic bioavailability of fatty acids (Ryan, 2018), particularly in the presence of higher obesity.It is shown that iron accelerates thrombosis due to its pro-oxidant mechanisms (Day, 2003), since the thrombotic effect of iron loads is inhibited by DL-cysteine scavenger (Franchini, 2008; Day, 2003). Thus, dietary iron intake may be associated with an increased risk of myocardial infarction, as reported 3 years ago in Finnish asymptomatic risk subjects. (Salonen, 1992) It has been widely proven that dietary iron restrictions confer protection against hypertension, cardiovascular remodeling and proteinuria in salt-sensitive Dahl rats (Naito, 2011); and all this mainly due to the inhibition of oxidative stress and the quantitative increase in the secretion of endothelial NOS synthetase. These investigations preclinically emphasize that the accumulation of metal contributes to the pathogenesis of cardiovascular diseases (Naito, 2011), particularly ischemic disease in non-diabetic subjects, as we will see below.ROS oxygen free radicals are largely involved in myocardial injury particularly that related to coronary artery occlusion followed by reperfusion. Transition metals - such as iron and copper - are necessary to catalyze the formation and lipid peroxidant action of ROS (Reddy, 1989). In the last two and a half decades, the crucial role of iron in the pathogenesis of myocardial necrosis has been fully demonstrated (Lesneksky, 1990). However, deferoxamine has only been proven to reduce (when administered concomitantly or later at the onset of reperfusion), but not to prevent iron-dependent myocardial cardiotoxicity (Reddy, 1991). Therefore, it can be concluded that: in iron-mediated inflammatory / oxidative processes, increased ROS formation contributes to the loss of myocardial viability throughout the ischemia-reperfusion process (Lesnefsky, 1990). Serum labile iron has been evidenced as a marker of the extent of acute vascular injury in acute myocardial infarction (and acute coronary syndromes), and the magnitude in its elevation independently forecasts prognosis (major adversecardiac events) (Lele, 2009; Lele, 213). And this is, in addition to promoting endothelial dysfunction, due to the direct ability of intracellular iron to firmly increase the adhesion of human monocytes to the endothelium (Kartikasari, 2004, Kartikasari, 2009), whose surface is dysfunctional. This would explain the prothrombotic action of parenteral iron (Day, 2003) under conditions of pre-existing arterial injury.Very interesting is the Nutrition Research Survey cohort study in Canada, where a clear association was found between serum free iron and the increased risk of fatal acute myocardial infarction (Morrison, 1994), but not in dietary iron: men and women with the highest category of iron in blood - equal to or greater than 175 ugr / dl had a higher risk of fatal myocardial infarction (95% confidence interval) (Morrison, 1994). We will point out that dietary iron was not considered an end-point in this work.Very interesting, among all subjects at high risk, the greatest amount of free iron is among the most serious smoking men in their coronary artery disease (Sharma, 2000).In another study with 496 subjects, 85 patients had clinically evident coronary disease (Rajapurkar, 2012); the measured catalytic serum iron (detectable iron bleomycin technique) in its most rangehigh was 10 times higher in subjects with greater extent and severity of their coronary heart disease (compared to patients with lower proportions of free iron. This demonstrates - again - evidence of the direct association between coronary heart disease and greater catalytic iron in serum, both in diabetics (Sulieman, 2004), prediabetic patients and patients with ischemic heart disease (Lele, 2013), as in the general population (Rajapurkar, 2012; Steen, 2013); and this takes on greater severity when this possible relationship of cause- Affection has an independent morbidity behavior.In addition, it has been shown that iron deposition at the level of cardiac tissue is pathophysiologically involved in the development of cardiac fibrosis induced by Angiotensin-2 (Ishizaka, 2002). And, in conditions of hypertensive stress (hemodynamic-metabolic overload), or what is the same, before an acute decompensation of blood pressure (hidden or not), iron overload is inducing the formation of neo-intimate and promoter of angiotensin-induced myocardial fibrosis (Ishizaka, 2002), and probably norepinephrine (Hashkova, 2011). This is proven by the fact that iron chelation protected both from induced catecolamine cardiotoxicity (mediated by ROS oxygen reactive species), and from iron injury. Iron depletion has been shown to reduce fibrosis of pancreatic islets in diabetic rats (Minamiyama, 2010).Thus, to date, two lines of evidence accumulate (experimental / preclinical / epidemiological) that demonstrate: 1) the crucial role of free iron (catalytic or labile): diseases where iron accumulates powerfully increase cardiotoxicity; and conversely:2): the protective effect of iron sequestering agents that reduce iron-induced cardiotoxicity; all of which is suggestive of a causal relationship (Thethi, 2011).Thus, as previously stated, the acute early treatment of iron chelation (Deferoxamine) has proven to reduce the extent and magnitude of cardiomyocyte necrosis, due to a two-hour episode of ischemiareperfusion (Reddy, 1989); and, although Reddy demonstrated the opposite in dogs (Reddy, 1991), another researcher showed that pre-treatment with Deferoxamine did decrease the magnitude and extent of heart attack by significantly reducing oxidative ischemia (Lesnefsky, 1990). However, Chan et al, specifically in a patient with heart attacks with ST-segment elevation (STEMI), deferoxamine did not achieve sufficient survival of myocardial tissue in the reperfusion period (Chan, 2012).On the other hand, today it is known that mitochondrial function, especially in cardiac tissue, is considerably affected both in insulin-deficiency and in insulin-resistance (Ovide-Bordeaux, 2003); and the latter worsens with iron in charge (Chi, 2013). In any case, since the function and structure of the heart muscle is affected, it is perfectly understood that the deficient action of insulin predisposes to cardiac dysfunction, by changing its muscular phenotype (Wang, 2003).Additionally, we know that endothelial-dependent vasodilation - and endothelial health in general - is affected by oxidative stress; in such a way that its increase mediated by iron modulates an adequate vascular tone negative (Houschyar, 2012).Finally, the generation of an excess of ROS directly promoted by free iron is inversely related to the effective signaling of insulin, resistance to its protective effects of the cardiovascular system (particularly endothelial) and associated functional cardiovascular pathology (Houschar, 2012).
It has been known for many years that clinical syndromes characterized by elevated iron accumulations, such as hemochromatosis and thalassemia, are associated with chronic myocardial dysfunction (Horwitz, 1999). Iron overload that occurs in subjects receiving frequent blood transfusions (betathalassemia major, myelodysplastic syndromes, sickle cell disease) causes multiple organ damage, however, it is the cardiac iron accumulation that leads to death (Wood JC, 2208 ). In cultured rat cardiomyocytes, a significant iron overload causes progressive loss of mitochondrial DNA and alteration in its respiratory chain, that is, a powerful cumulative and irreversible oxidative damage (Gao, 2009).A physiological concept that becomes pathophysiological over time must be very clear: iron has no mechanisms of excretion and real elimination (Lukinova, 2009; Pietrangelo, 2003). Physiological insulin, especially excessive, increases body iron deposits such as ferritin, and this acquires its highest relevance in diabetes (Fernández-Real, 2002). Today it is shown that the presence of type 2 diabetes in the general population is independently associated with the increase in all causes of mortality in patients with ventricular tachyarrhythmias upon admission (Weidner, 2018). This is corroborated by the ignored increase in sudden death in diabetics (Andrássy, 2008, rev) - precipitated by hyperglycemia that prolongs the QT interval (see later).The first evidence in humans is greater than two decades: it was found that a high serum ferritin (greater than 200 micrograms / lt) was a powerful risk factor for acute myocardial infarction (compared to low quintiles) in male subjects at risk, and asymptomatic coronaries; and, as is now understood, this association was much closer in those subjects with greater insulin resistance - that is, with lower HDL (Salonen, 1992). Dietary iron intake has shown a significant association with an increased risk of getting sick (Fernández-Real, 2002). Long-term complications of iron intravenous therapy are known in subjects with end-stage renal disease, especially galloping atherosclerosis with increased risk of infection, particularly in subjects with metal overload (Sengoelge, 2005). It has been reported in Mexican children and adolescents with end-stage renal failure, an increase in cardiovascular risk factors (C-Reactive Protein and Interleukin-6) with iron overloads, and in direct relation to Ferritin levels). Parenteral iron affects the structure and cardiac function of metal deposits in the myocardium (Ruiz-Jaramillo, 2011) and increases the early progression of atherosclerosis (Wollf, 2004, Drüeke, 2002). In addition, it has been shown in vitro that iron induces calcification of smooth vascular muscle cells of the aorta, directly, and in the absence of TNFalfa (Kawada, 2018). Free iron (not bound to transferrin: non-tranferrin-boun-iron) present in human serum is directly pro-oxidant and inflammatory cell and vascular, since it activates the endothelium (increasing pre-existing endothelial dysfunction) by increasing molecules of adhesion and pathologically elevate the monocyte-endothelial interaction (Kartikasari, 2004; Kartikasari, 2006; Kartikasari, 2009).We can categorically state that accumulated iron increases the risk of Carotid Atherosclerosis (Kiechl, 1997), specifically by elevating the soluble endothelial adhesion molecule ICAM (intercellular-adhesionmolecule), which has a powerful predictive value in the progression of atherosclerosis, and independently of the classic cardiovascular risk factors (Kondo, 2005).In an interesting prospective study with more than 13 thousand women in 10 years of follow-up, it has been revealed that a high prenatal dietary intake of Iron Hem is associated with an increased risk of acquiring gestational diabetes (Bowers, 2011), but the highest Public health relevance is that serum ferritin correlates directly and powerfully with glycidic intolerance (Lao, 2001). Obese women with high serum ferritins havegreater mobilization of fatty acids, greater lipolysis in adipose tissue and greater insulin resistance compared to their low ferritin pairs (Ryan, 2018).It is interesting to clarify the type of coronary artery disease that is closely related to ferritin excesses: in relation to obstructive coronary pathology (defined as more than 50% stenosis in the diameter of at least one coronary artery) no it is related to ferritin levels in both sexes (Auer, 2002); however, this does appear to occur in non-obstructive inflammatory coronary disease (coronary syndrome X).Recently it has been demonstrated once again that high levels of ferritin are a clear risk factor for carotid atherosclerosis (Wolff, 2004) in women and for coronary disease in men (Won, 2011). Conversely, in cell cultures, isolated organs and preclinical studies it is evident that depletion of iron deposits prevents restenosis, reperfusion injury and atherogenesis (Horwitz, 1999).Ferritin concentrations are closely associated with the presence of subclinical early atherosclerosis (measured by the coronary concentration ofcalcium), and completely independent of diabetes and other cardiovascular risk factors (Sung, 2012). And again, conversely, normal low levels of tissue (and circulating) iron can reduce oxidative stress and its markers in healthy adults (Mendes, 2009).Iron-Zinc micronutrient balance is very interesting, particularly in the dyslipidemic subject: if the zinc intake was as generous as the daily and excessive iron intake, the toxicity of accumulated iron could be reduced (Wong, 2007; Song, 2013). But, this does not happen in a classic western diet.Today we know that the highest concentrations of ferritin are associated with a presence of early coronary atherosclerosis markers, and independently (You, 2005, Sung, 2012); and this becomes more relevant in subjects with genetic insulin resistance identical to ours (You, 2005).In an longitudinal study of more than 7,000 Asian men, an association of elevated serum ferritin levels with a higher incidental risk of hypertension has been reported: thus, subjects with high baseline ferritin levels will develop HT (Ryoo, 2015). Serum ferritin emerges as an early marker of cardiovascular risk, particularly in healthy oriental men with direct diabetic family rank (Liu JR, 2019); and in an extensive meta-analysis, together with high triglycerides, ferritin was a clear and early component of the metabolic syndrome (Suárez-Ortegón, 2018).
The excessive use of glucose due to a deregulation in its metabolismpromotes inflammation in monocytes and macrophages in subjects with atherosclerotic coronary artery disease (Shirai, 2016, Ieronimaki, 2019b). Today we know that the master hormone in modulation of innate immunity is Insulin (Ieronimaki, 2019a, Ieronimaki, 2019b)The elevation of triglycerides accompanied by the reduction of HDL cholesterol, hyperinsulinemia and fibrinolytic alteration, all this is found in subjects with premature coronary artery disease, as a previous reflection of insulin resistance syndrome (Bávenholm, 1993, Jovinge, 1998). Especially in men with ischemic heart disease there are significant increases in insulin and triglycerides, the latterstatistically independent, persisting as the only univariate risk for cardiovascular disease (Yarnell, 1994). Very interesting is the extensive Italian study that shows that in young men, the increase in triglycerides and uric acid - directly related and cardiovascular risk factors - occurs independently of central obesity and abdominal adiposity (Bonora, 1996).An electrocardiographic data that can predict sudden death can be the QT interval: its prolongation (corrected) represents a delay in the depolarization of the ventricular myocardium, being able to be a precursor of malignant arrhythmias and sudden death (Munger, 1991, Moss, 1993). Since the QT interval has recently been associated with metabolic syndrome (Ravikumar, 2017, Dekker, 1996, Park, 1997, Nishizaki, 2002, Rautaharju, 1994), today it is shown that Insulin Resistance affects myocardial activation, it can increase the QT interval and increase the risk of sudden death (Shin, 2005). In obese women, especially those with visceral adiposity, a progressive decrease in weight, improves cardiac repolarization time, significantly shortening the QT interval, reducing the risk of electrical ventricular instability; and this in strict correlation with the reduction of FFA-free fatty acids (Colbi, 2002), which substantially increase insulin resistance. And its increase, even in normal subjects, prolongs cardiac repolarization, (Marfella, 2001). It is known that lengthening the QT interval has been particularly associated with abdominal obesity (Peiris, 1991, Park, 1997, Ravikumar, 2017). This is consistent with the finding that hyperinsulinemia would be associated with the presentation of premature ventricular complexes (Paolisso, 1996).Normal physiological concentrations of insulin would not prolong the QT Interval on their own: as recently reported, only late after an insulin clamp in healthy subjects would the QTc interval be significantly increased (Taubel, 2012), and this should be a fluctuation and decrease in serum potassium (Gastaldelli, 2010, Taubel, 2012). But ç, in insulinresistant subjects, the prolongation of QTc would be due to both rapid fluctuations of potassium and those of basal hyperinsulinemia (see above).On the other hand, the presence of iron, particularly in its unbound form, is decisive in the production of free radicals, and especially in its acceleration. Thus, it has been shown that the increase in iron deposits is closely associated with greater severity in vascular lesions of humans and animals (Ishizaka, 2002).It has been recently pointed out with experimental bases that excess free iron rapidly causes arterial inflammation because it generates insoluble fibrin - parafibrin, which when deposited in the vascular wall initiates inflammatory reactions (Lipinsky, 2013-c). Likewise, the greater bioavailability of free iron can directly increase platelet activation and increase the risk of myocardial infarction in subjects with risk factors, by favoring the fissure or rupture of an unstable plaque (Praticó, 1999). In this way, it is explained that iron is potentially prothrombotic (Day, 2003). Magnetic Resonance Spectroscopy has shown that arterial iron promotes plaque instability (Pisano, 2016, rev), and increases its risk of rupture, especially in the presence of previous Vascular Insulin resistance (see Figure 9).The probable association between iron deposits and at risk of coronary heart disease was suggested for 4 years in a large Finnish study, which reported a clear interrelationship between the iron body status as ferritin, and dietary iron with the increased risk of heart attack. Myocardium in men (Salonen, 1992).In relation to sudden cardiac death, it has been shown that it occurs at a high frequency in very young subjects with hemochromatosis without any mutation (Klintschar, 2008). In this regard, it has been repeatedly demonstrated that young subjects with greater thalassemia have greater variability in repolarization and in the QT interval indices, probably due to cardiac iron deposits (Magri, 2007).Based on repeated clinical evidence, for more than a decade, the prolongation of the QT interval is considered to be a powerful indicator of increased risk of malignant ventricular arrhythmias and sudden death (Veglio, 2004), especially in the diabetic subject (Veglio, 1999).The prolongation of the QT interval (corrected for heart rate) is associated with the elevation of sudden death from coronary heart disease, and even in the absence of it (nocturnal ventricular fibrillation) (Munger, 1991, Browne, 1983): and the prolongation QT interval is a current part of insulin resistance syndrome (Dekker, 1996). Ventricular instability due to greater variability in the QT interval (alteration in myocardial repolarization) is clearly associated with a propensity for sudden death due to ventricular malignant arrhythmias (Piccirillo, 2007).This dangerous association can be explained not only by the greater sympathetic activity induced by high insulin levels (Dekker, 1996), but by the greater oxidationcoronary artery bioavailable iron: thus, the occurrence of arrhythmias can be exacerbated with electrolyte change coupled with a greater iron deposit, all of which can lead to sudden death (Shamssedin, 2011).In addition, any prolongation of the QTc interval (alterations of ventricular repolarization) is associated with a high and unexpected mortality in diabetic populations (Weston, 1997).In an interesting case-control study of older individuals, hyperinsulinemia (and especially glycid intolerance) has been shown to be strongly associated with a significant increase in the QTc interval (and a concomitant shortening of the RR interval due to sympathetic hyperactivity); and both phenomena: the shortening of the RR and the prolongation of the QTc are strongly associated with the increased risk of sudden death (Van Noord, 2010).Apparently, as reported in a very interesting study, the QTc interval is longer in women (when compared to values in men). It has been categorically stated that, particularly in normoglycemic women, insulin resistance is a powerful independent determinant of QTc prolongation (Shin, 2005). In addition, of great interest is the report by Marfella et al. which shows in normal men that acute hyperglycemia in high physiological ranges causes a significant increase in the QTc interval and its normal dispersion (Marfella, 2000).In non-diabetic, non-hypertensive subjects, without cardiac hypertrophy or coronary pathology, it has been shown that excess fasting insulin is strongly correlated with the frequency of premature ventricular complexes (Paolisso, 1996).On the other hand, it was demonstrated in early type 2 diabetic subjects with angiographically normal coronary arteries that low doses of deferoxamine, a powerful iron chelator, totally restore the altered coronary flow during stress (sympathetic stimulation by the cold pressor test) (Nitemberg , 2002). This would once again indicate the reducing role of cardiac tissue iron in the optimal release to sympathetic stress of nitric oxide. In addition, in conditions of acute stress, free iron can potentially reduce coronary angiogenesis, since its chelation with dexferroxamine has been shown to increase it significantly (Chekanov, 2002).Ferric ions can activate coagulation and induce the formation of dense fibrin deposits, as occurs in diabetes or Stroke: thus, iron loads or their larger deposits would induce spontaneous fibrin formations (Pretorius, 2013).In sudden infant death, iron in charge could have etiological involvement (Moore, 1989; Weinberg, 2001), since the coincidence of death with itsunusual concentration in the liver has been demonstrated (Moore, 1994); and liver iron would be of exogenous origin, since umbilical cord ferritin does not increase in victims (Raha-Chowdhury, 1996). These sudden deaths would occur in babies with excess insulin, since their incidence is almost nil in Asian populations (Moore, 1989).It is extremely interesting that it has been shown that iron overload in rodents is the cause of selective alterations in cardiac electrical conduction (Schwartz, 2002; Rose, 2011) and atrial fibrillation (Rose, 2011). Thus, a bradycardia in a normal heart as a product of burdens
Sub-acute or chronic iron (Sellan, 2009; Rose, 2011, Siri-Angkul, 2018) in the presence of a prolongation of the QT interval, would be doubly fatal.In addition, in an interesting study of almost 2000 men and women in China, it was found that patients with metabolic syndrome have a prolongation of this corrected QT interval (Li, 2008). Pathophysiologically, high iron, by reducing insulin sensitivity, would also alter the autonomic cardiac tone, since this hormone is pivotal in the autonomic regulation of heart rate control, directly and through parasympathetic tone (Bergholm, 2001). This Metabolic Autonomic Neuropathy - a non-infrequent promoter of fatal arrhythmias in pre-diabetics and diabetics - and which, not only responds, but causes itself, greater tissue inflammation (Vinik, 2012, Vinik, 2013, Herder, 2017), could be increased by rapid increases in cellular and dietary iron.The incidence of unstable angina, myocardial infarction, cerebrovascular accidents, pulmonary thromboembolism, and sudden deaths, presents a significant daytime variation, with its presentation at dawn and this due to the greater morning platelet aggregation (Manfredini, 1996, Muller, 1989, Tofler, 1987), because of the nocturnal increase in insulin resistance (which alters fibrinolysis, procoagulant activity, endothelial function and blood pressure); and this accentuation in insulin resistance, in turn, would be electrolyte and iron-dependent (see above). This hypofibrinolysis is increased more and adds to hypercoagulation - in vitro and in vivo - due to an increase in free iron in the blood (Kell, 2015, Lipinki, 2012, Lipinski, 2012b) due to systemic inflammation (Lipinski, 2013, Lipinski, 2013b), which reaches its maximum hypercoagulant expression in type 2 Diabetes mellitus (Randeria, 2019, Buys, 2013).It is little known, but solidly epidemiologically evident (ARIC Study - Atherosclerosis Risk in Communities) - that thin hyperinsulinemic subjects have higher atherogenic risk factors, compared with obese subjects - particularly diastolic tension and uric acid - (Nabulsi, 1995, Bonora, 1996, Bonora, 1987). We postulate that this is due to the increased tissue accumulation (and dietary intake) of iron. In this regard, it is very interesting the finding in genetically obese mice that elevated iron reduces visceral fat, by promoting small adipocytes with large metal accumulation and severe insulin resistance (Ma, 2018), promoter of diabetes (see later).
In a magnificent and long European prospective study, it has been shown that greater body deposits of iron, measured as serum ferritin, are significantly associated with the increased risk of diabetes, and independently of classical factors (Montonen, 2012). Therapeutic doses of intravenous iron cause high oxidative stress and necrosis of beta-pancreatic cells of rodents (Masuda, 2014) and apoptosis of human polymorphonuclear (Ichii, 2012). In hepatocytes, intracellular iron loads reduce the protein levels of IRS1 and IRS2 Insulin receptors, causing Insulin resistance (Varghese, 2018).Hemoglobin levels are increased in the cardiomyocytes of patients with terminal heart failure, despite global mitochondrial dysfunction, which would lead them more rapidly to cell death, as reported recently (Khechaduri, 2013). Acute or chronic myocardial damage due to ischemia / reperfusion is prevented in vivo by capturing accumulated mitochondrial iron and non-apoptotic cell death - ferroptosis, according to an interesting recent report (Fang, 2019).Hemoglobin is directly related to the severity of insulin resistance in non-obese men and women: hematocrit is increased in insulin resistance (Facchini, 1998; Houschyar, 2011) or in any subject with abdominal obesity. It has been shown in humans that high non-physiological levels of hemoglobin accelerate the atherosclerotic process (Marx, 2008). Also, excess iron in hemoglobin could be powerfully implicated in the genesis of gestational diabetes (Lao, 2001).Thus, in an ambitious meta-analysis of 16 years and 33 studies, biomarkers of dietary iron (hemoglobin, ferritin, transferrin saturation) are higher in women with Gestational Diabetes (Kataria, 2018), which is reinforced in another meta- Previous analysis, in particular on the greater contribution of Hem iron and the significant risk of diabetes - RR is increased 1.38% per mg. of increase in dietary iron - (Zhao, 2017).Although diabetes and cardiovascular pathology, particularly ischemic, share many risk factors and pathophysiological mechanisms, the primary causes for Diabetes 2 include, in addition to IR, a progressive beta cell dysfunction: today it is known that both processes are due potentially to the direct effects of accumulated iron. These elevated deposits such as ferritin accumulate in the liver, muscle and pancreas cause organ-specific oxidative damage leading to diabetes (Jiang, 2004- a and b).A decade and a half ago, evidence emerged that high blood viscosity and increased hematocrit are clear risk factors for severe insulin resistance and type 2 diabetes (Tamaris, 1998). In a long sample of non-diabetic subjects with metabolic risk factorsa significant association of the hematological parameters was found with the reduction in insulin sensitivity and the dysfunction of the beta cells of the pancreas (Hanley, 2009).It has recently been shown that the increase in intracellular iron in human adipocytes (and the Heme molecule) alters the systemic glucose metabolism (Moreno-Navarrete, 2017).Thus, four studies have demonstrated the prospective association between elevated hematocrit or hemoglobin and the subsequent occurrence of type 2 diabetes mellitus (DM2); and this could only be explained by the fact that a high viscosity of the blood reduces powerful and particularly the microvascular flow contributing to a tissue insulin resistance (Tamaris, 1998; Stuart, 1980). However, it has recently been shown that it is high hemoglobin without a rise in viscosity that would play a preponderant role in slowing coronary flow in nonobstructive coronary artery disease - unstable blood - (Bilgi, 2013). For example, in British men, Wannamethee found (Wannamethee, 1996) that a high hematocrit had a strong and graduated correlation with the incidence of DM 2 after 12 years of follow-up; Medalie et al. Found a direct association between the hemoglobin of middle-aged men and the incidence of diabetes (8688 subjects). In the Framingham-Framinghan Heart Study, hemoglobin predicted a glucose intolerance in women. And recently, Tulloch found that the Hematocrit was related to the incidence and severity of diabetes 2 among more than 1200 Pima Indians, after a followup of 11 years.It has recently been confirmed in an interesting cross-sectional study in the Asian population that subclinical iron overloads -measures such as ferritin- are associated with increased insulin resistance, especially in men (Kim, 2011). High ferritins are associated with early atherosclerosis due to the presence of their systemic and tissue markers; but most importantly, independently of the traditional risk factors (Score Framinghan; diabetes, and insulin resistance - dysmetabolism, vasculopathies and pre-existing low-grade inflammation) (Sung, 2012).Ferritin, today, is considered a proinflammatory cytokine (Rudell, 2009), by clearly stimulating the inflammatory transcription factor NfKB. It has been reported with experimental evidence that high iron isnot only a risk factor but is a causal factor for Insulin Resistance syndrome (Metabolic syndrome) (Gabrielsen, 2012), particularly for its inhibitory effect on transcription of Adiponectin, the only hormone that increases insulin sensitivity. In this regard, the inverse correlation between ferritin levels and adiponectin levels is known lately, especially in diabetic individuals (Ku, 2009; Forouhi, 2007). Thus, high Ferritin figures - in “normal” rangesindicated a 7 times higher risk (compared with low ferritins) of diabetes (Forouhi, 2007).Diabetic subjects with nonhypertensive diabetic retinopathy have much higher levels of ferritin than those with hypertension; but the most relevant is that the severity of diabetic retinopathy correlated with serum ferritin values (Coban, 2010). At higher concentrationsof ferritin, the greater the severity of diabetic complications: in women, higher levels of accumulated iron increase the severity of insulin resistance in diabetes 2 (Yu, 2012). It has been shown that the prothrombotic state of diabetes, as well as its cardiovascular complications, would be explained in terms of a chronic in vivo persistence of the oxidative-inflammatory actions of free iron (Lipinsky, 2012).However, we must clarify this concept: in a subject with an ideal insulin in quality and quantity (100% sensitivity), it is almost certain that initially an iron overload does NOT directly or rapidly cause insulin resistance (Abraham, 2006); nevertheless, this is, in modern life, unreal: we all have a greater or lesser degree of tissue insulin resistance (Jara, 2003); and nobody ingests only isolated iron, because it always comes in combination (in particular with fats). Thus, dietary iron is an inducer and promoter of insulin resistance in the current subject (Fernández-Real, 2002) and promoter of dyslipidemia and metabolic syndrome (Lin, 2018); and first, immediately: it has been shown that, through increased hepcidin secretion, iron in the diet correlates positively with the aggregation of erythrocytes (Lin, 2018).Tissue iron levels are determinants in the secretion and action of insulin (Abraham, 2006; Fernánde-Real, 2002). Thus, several lines of evidence strongly suggest that iron has a preponderant role in the pathogenesis of type-2 diabetes, both because it directly undermines the action of insulin (FernándezReal, 2002; Ford, 1999, Tuomainen, 1997, Jiang, 2004-a; Jiang, 2004-b), as selectively toxic on pancreatic beta cells (Lu, 1994; Kishimoto, 2010).Thus, acting directly for its cumulative toxic effect on pancreatic beta cells, iron is a factor that determines the pancreatic capacity in insulin secretion (Cooksey, 2010; Abraham, 2006), and, as we have already pointed out, the degree of Your numbnessAll states of iron overload, but above all the “normal” levels of body iron that become with the passage of time in a “physiological” overload contribute decisively to cardiovascular pathology; and this is demonstrated by the accumulation of evidence: iron chelation continues to demonstrate the ability to prevent coronary restenosis (Horwitz, 1999). Let us not forget that the combined excess of insulin, but particularly leptin, is closely associated with a high incidence of stent restenosis (Piatti, 2003; Schafer, 2004).It is shown that ferritins greater than 600mg% in hemodialysis patients have a high mortality at 4 years of followup, due to a marked increase in systemic inflammation - measured by PCR - in the absence of infection (Kletzmayr, 2002). And in these populations, a high catalytic iron is associated with a greater presence of significant coronary pathology (Rajapurkar, 2013).It is important to point out that cardiomyopathy is caused by the deposition of cardiac tissue iron - not ischemic coronary disease - the cause of death in terminal hemochromatosis; the extreme frequency of diabetes in these iron overloads, added to the fact of the substantial improvement that iron-reducing therapies exert on glucose intolerance (and insulin resistance), all of this provides remarkable evidence that the iron body deposit participates directly on the origin of type 2 diabetes in the general healthy population (Jiang, 2004b).Iron is a potent pro-oxidant, and its increased levels increase tissue and vascular oxidative stress: increasing epidemiological evidence reinforces a positive association between high levels of circulating ferritin and the increased risk of all insulin resistance states (metabolic syndrome, gestational diabetes, ovarian polycystic disease).Cumulatively, important clinical studies continue to show that phlebotomy or blood donation are capable of improving sensitivity (Valenti, 2007, Rajpathak, 2009) or insulin secretion (Abraham, 2006) in humans. It has even been reported that this reduced insulin secretion can be reversed by phlebotomies in animals (Cooksey, 2010) and humans (Abraham, 2006).Recently, the presence of an arrhythmogenesis has been demonstrated in diabetic heart failure due to an alteration in cardiac electrophysiology (reduction in Ito current) due to poor insulin signaling in cardiomyocytes (Lengyel 2007, Dubó, 2016): the sudden sudden death would be precipitated by an acute release of catalytic iron, in the context of an abnormal repolarization?. In this regard, it has recently been shown that the genetic loss (or reduction) of insulin signaling in cardiac cells accelerates left ventricular dysfunction subsequent to myocardial infarction (Sena, 2009, rev).In a recent meta-analysis published (Orban, 2014), it is shown that ferritin and the clinical elevation of transferrin - even independently of the inflammatory variables - are associated, suggesting a causal effect of type 2 diabetes. In another magnificent review (Hansen, 2014, rev), it is shown that excess iron causes inflammation (direct and cytokine-induced) and subsequent apoptosis of pancreatic beta cells; and is key in the pathogenesis of diabetes 1 and 2.It has just been demonstrated, again, but now in db / db mice that dietary excesses of iron increase gluconeogenesis, reduce lipogenesis (by reducing visceral fat), adiponectin levels, increasing insulin resistance (liver and fat) and glucose in fasting (Lin, 2013, Ma, 2018). In this regard, Adiponectin - the only anti-inflammatory and anti-atherosclerotic hormone - has been shown to reduce, experimentally, cardiomyopathy induced by iron overload (Lin, 2013).Very interesting, every patient with hemochromatosis subsequently has diabetes, unless he has been treated with regular phlebotomies, which can reverse the vascular disorder and improve dysglicemia (Wolff, 1993, Basuli, 2014, rev; Vinchi, 2014, rev). On the other hand, if phlebotomy improves the enzymology and histology of the liver in subjects with Non-Alcoholic Liver Disease (Khodadoostan, 2017), it should be recommended in patients with hepatic steatosis: excessive body iron is associated with an increased risk of fibrosis. Hepatic, Cardiovascular Disorders and Cancer (Guillygomarc’h, 2001). Moreover, if bimonthly phlebotomies have been shown to reduce advanced fibrosis and cirrhosis (Kowdley, 2016, Mehta, 2019, rev), could they be early and therapically beneficial in Cardiac Fibrosis?
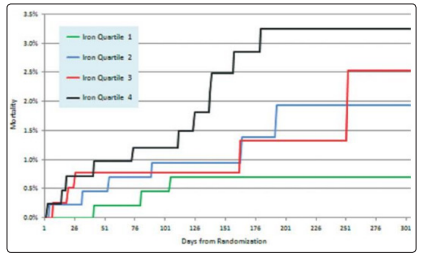
Figure 1: Kaplan-Meier curves that analyze general Mortality by Quartiles,according to the level of the Catalytic Iron(Steen,2013)

Figure 2: Effects of Chronic Iron Overload on Heart Rate (Conscious Mice): its bradycardizing effect. (See explanation in: Rose. 2011)
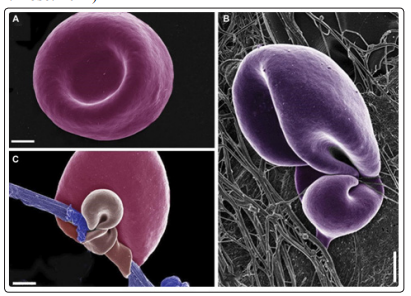
Figure 3: Ferric ions alter normal morphology of Erythrocytes A) Red blood cells show their typical physiological form in nondiabetic subjects with ferritin and transferrin in normal ranges (10 - 120 ng / ml, and 2.53.8 gr / l, respectively)B) Unusual configuration of fibrin fibers interacting with Erythrocytes in diabetic subjects with Ferritin and Transferrin slightly elevated C) Erythrocytes of normal subjects, in whose whole blood very low concentrations of Ferric Chloride (0.03mm) similar to physiological levels of ferric overload were added , which promotes the deformation of red blood cells by being their cell membranes trapped by altered fibrin fibers (parafibrin)Excessive iron is prothrombotic(Taken and in simplified text from: Pretorius, 2013 a)
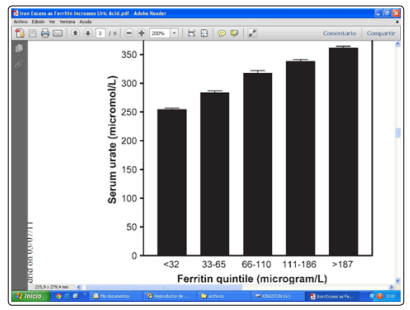
Figure 4: Effects of Ferritin concentrations on average serum uric acid concentrations (in 9726 individuals without any chronic inflammatory conditions) and consistent with the ferritin quintile values (Ghio, 2004)
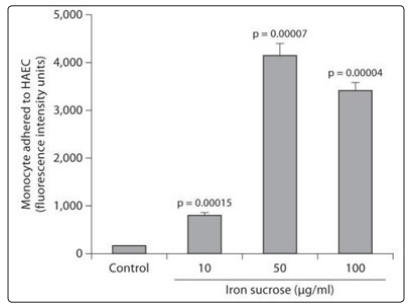
Figure 5: Significant increase in monocyte cell adhesion to cultured endothelial cells, due to Iron-Sucrose, reflecting a deep iron-induced prothrombotic endothelial dysfunction(Modified text of the graphic of: Kamanna, 2012)
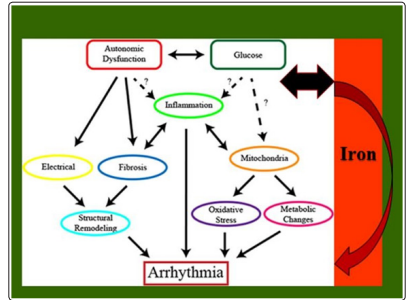
Figure 6: It is shown how acute Glucose fluctuations, enhanced by metabolic autonomic dysfunction, promote inflammation, increased oxidative stress and fibrosisCardiac, mitochondrial energy damage, all of which promotes electrical disturbances,(electro-metabolic) and generates arrhythmias; all of which would be enhanced, and in acute form, by the catalytic ironIt begins to show that Iron inoverloads, by causing inflammation, metabolic, autonomic and electrical disturbances can trigger Fatal Arrhythmias Graph taken (Text added in italics) from: Grisanti, 2018
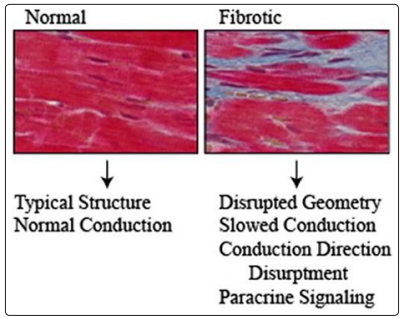
Figure 7: Histology of a myocardium with diabetesCardiac Fibrosis and structural changes in the geometry of the heart, which occur in Diabetes, are conduction disorder generators and promoters of arrhythmias, in conjunction with iron and fat deposits (not visualized here).(Fibrosis in blue, cardiomyocytes in red) (Figure Taken with modified text from: Grisanti, 2018, rev).It is the function that determines the vascular structure (Mather, 2004, rev).
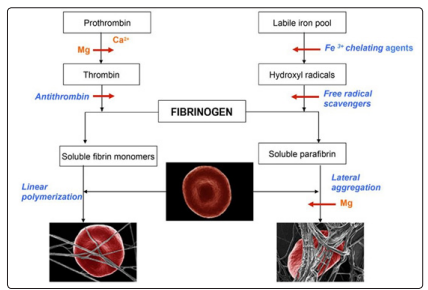
Figure 8: Schematic representation showing the biochemical events that lead to the formation of a normal clot - fibrin monomer formation (left panel) and the pathological formation of the clot by induction of parafibrin in the presence of iron(Right panel) (Graph taken from: Lipinski, 2013, rev (d))
This non-enzymatic formation of Insoluble Fibrin (Parafibrin) due to excessive free iron in the blood is of enormous clinical importance: in addition to reducing thehemostasis and physiological fibrinolysis, the deposition of parafibrin in the arterial wall will cause an inflammatory and prothrombotic reaction (Lipinski, 2013, c).
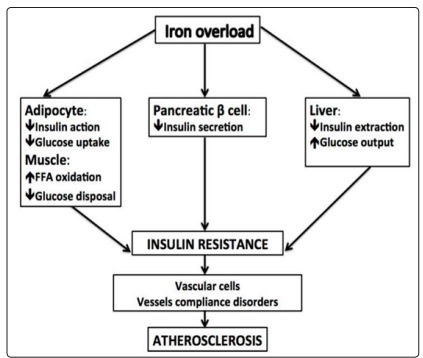
Figure 9: Scheme showing the direct effect of “common” dietary iron overload on the (rapid and cumulative) generation of Insulin Tissue Resistance (adipose,hepatic and pancreatic), which, in turn, causes acute vascular dysfunction, and promotes increased endothelial inflammation and atherosclerosis (with increased acute atherothrombotic disorders(Figure taken with modified text from: Pisano, 2016, rev)
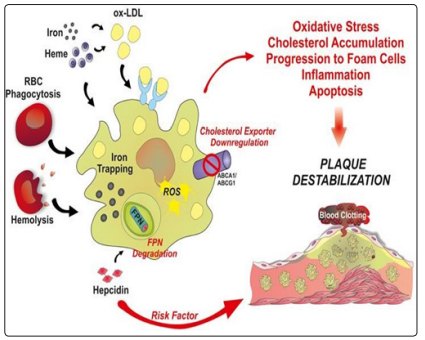
Figure 10: The effect of rapidly released iron (hemolysis) or sequestered (exogenous acute iron) is shown; its oxidative and inflammatory vascular effect, which increases the progression and acute inflammation within an atheroma plaque. Particularly relevant is the deleterious effect of exogenous iron loads in subjectswith high hemoglobins, by sharply raising hepcidin, which increases iron sequestration in macrophages, causing an acute increase in inflammation.This acute inflammation causes greater apoptosis of the plate stabilizing muscle cells, precipitating their rupture and promoting an acute thrombotic event, and the risk of sudden death. (Figure taken, expanded and modified text from: Vinchi, 2014)
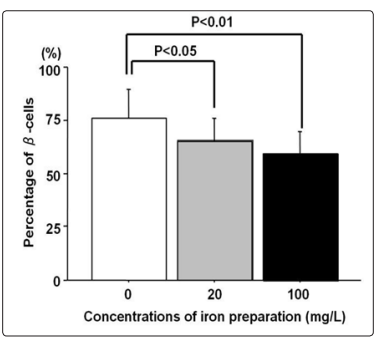
Figure 11: The effect of sucrose iron on the amount of beta cells in pancreas islets is shownAs the dose of sucrose iron increases, a significant decline in the percentage of pancreatic beta cells is observedThe toxic cellular effect of therapeutic intravenous iron on the beta pancreatic reserve and its ability to cause insulin deficiency is demonstrated (Taken from: Masuda, 2014) After the publication of this review, it is evidenced that the decrease in amounts of iron in pancreatic tissues decreases blood glucose in diabetic animals and cell models (Shu, 2019)
An obvious atherogenic role of iron: an increase in 10mg. of serum ferritin levels increases the 3% probability of having at least two atherosclerotic plaques (Ahluwalia, 2010) in middleaged men. The epidemiological, histological, experimental and preclinical evidence is overwhelming: atherosclerosis is the vascular expression of a chronic iron toxicity (Zachaski, 2003). Stadler et al. They directly quantified iron deposition and ferritin induction in early lesions - in endothelium and macrophages - and in late lesions - in smooth vascular muscle cells -, additionally (Stadler, 2004; Vinchi, 2014, rev).
Atherosclerotic lesions in human carotids contain up to 17 times more iron than control arteries, while iron restriction is associated with the disappearance of medullary tissue iron (Stadler, 2004; Sullivan, 2008).The increase in macrophage iron levels is decisively promoter of atherogenesis, increasing the oxidation and accumulation of LDL-cholesterol, as demonstrated by Kraml (Kraml, 2005). In numerous animal models of atherosclerosis, it has been shown that the reduction in vascular iron content - as a result of a dietary restriction - significantly reduces vascular oxidative injury and atheroma formation (Lee, 1999).With the current evidence on the rise in animals and humans, there is direct confirmatory evidence of the causal involvement of iron at the onset of atherogenesis (Ponraj, 1999). Iron deposits are evident in human atherosclerotic lesions, and have a close causal association with the progression of atherosclerosis.In primary and particularly secondary prevention, the current physician should know that an antioxidant anti-inflammatory treatment is of no use if the iron tissue stores are elevated, as has been determined in numerous investigations on the vicious progression of coronary atherosclerosis (Sullivan, 2004).Exogenous restriction of dietary iron decreases atheroma inflammation by reducing the expression of MMP-9 metalloproteinase enzymes (Lee, 2003); and concomitantly iron exogenous restriction has been shown to increase the collagen content of atherosclerotic lesions, all of which implies that dietary iron restriction potentially reduces inflammation and instability of atheroma (Lee, 2003). Thus, restriction of exogenous iron would significantly inhibit the formation of atheroma by reducing its inflammation; with metal restriction, the proportion of iron-oxidized LDL-cholesterol would be reducedconsiderably (Lee, 1999). In the interesting Pomeranian study, there is a strong direct relationship between high serum ferritins and carotid atherosclerosis (Wolff, 2004).As for the accumulated iron, today it is known that, in addition to activating the transcription factor NfKB, of high inflammatory and fibrotic potency, ferritin, which behaves like a true inflammatory cytokine, stimulates increased activity of interleukin IL-1b (Rudell, 2009). Recall that systemic inflammation is a risk factor for heart disease (Kaplan, 2001), just as vascular inflammation is profoundly for atherosclerosis (Libby, 2002); accelerating the atherosclerotic process in the presence of labile iron (Kruszewski, 2004).An interesting in vitro study reliably reveals that IronSucrose (commonly used in intravenous preparations) causes significant endothelial injury, dose / time dependent, due to its marked reduction in acetylcholine-dependent arterial relaxation (Kamanna, 2012).Definitely, the accumulation of iron (and copper) is capable of oxidatively damaging the extracellular matrix and, especially, high iron values can inevitably affect the stability of the atherosclerotic plaque and promote its rupture (Stadler, 2004). The known therapeutic failure of known antioxidants on all degenerative prothrombotic diseases is due to chronic overload in iron deposits or acute load in free iron, due to its direct and powerful thrombosis and coagulation activating capacity in stress (Pretorius, 2013b; Day, 2003; Lipinsky, 2012b). Recently, a synergistic association between ferritin and cholesterol levels has been demonstrated in a very representative sample of American men and women post-menopausal (Menke, 2009).Thus, the reduction in body iron deposits and the chelation of their ions have beneficial effects on the development of atherosclerosis, and potentially decrease the formation of post-ischemic lesions (Kruszewski, 2004).
Especially in subjects with high hemoglobin (Potor, 2013, rev), acute iron loads would cause or exacerbate an acute instability of atheroma by causing an immediate increase in Hepcidin Hormone and increased intracellular iron sequestration: both, High Catalytic Iron and Hepcidin would promote acute instability in atheromatous plaque and consecutive acute thrombosis (Vinchi, 2014, rev). The discovery that pancreatic beta cells express and secrete physiologically Hepcidin to iron or glucose loads is extremely interesting (Kulaksiz, 2008). This would explain that in hereditary hemochromatosis - genetic deficit ofHepcidin- (not in secondary to transfusions) there is relative protection against accelerated atherosclerosis (See Figure 10).
Extremely worrying is the current epidemic of abdominal adiposity (visceral obesity) since childhood, which has been shown to present a high risk of sudden death from high iron intakes, which promotes severe resistance (Steinberger, 2003) vascular to insulin. Extensive epidemiological studies have shown that frequent blood donation is associated with a decrease in the risk of acquiring type 2 diabetes and Coronary Artery Disease (Fernández-Real, 2005, rev; Vinchi, 2014, rev, Manco, 2012, rev), reducing substantially the risk of atherosclerotic cardiovascular events, especially in non-smoking men or with high LDL (Meyers, 1997, Meyers, 2002, Vinchi, 2014, rev). Cholesterol levels in atherosclerotic lesions correlate directly and closely with their iron deposits(Stadler, 2004; Vinchi, 2014, rev).The fact that tissue chelation significantly reduces the size of myocardial infarction after occlusion is conclusive only when the chelator (deferoxamine) is administered regionally (Kobayashi, 1991). This is consistent with what has been known for many years: reactive oxygen species are not very powerful in their damage in the absence of iron (Horwitz, 1999).”Normal” iron and fat, joint overloads, cause mitochondrial dysfunction, profound insulin resistance, and lower levels of antioxidant HDL cholesterol (Choi, 2013). Even in the absence of iron overloads, this metal, one of the most pro-oxidation that exists, and that accumulates physiologically, causes disturbances in glycidic metabolism (Fernández-Real, 2002).The progressive accumulation of iron that results in body overload can cause tissue resistance to the biological effects of insulin (Fernández-Real, 2002), and deficiency in insulin secretion, but this often does not result in diabetes (Cooksey, 2010), but in the Insulin Resistance syndrome, the silent millennium epidemic.In this regard, more than two decades ago, Zidek et al. Demonstrated that repeated phlebotomies resolved resistant hypertension (with triple medication) (Zidek, 1985). Ina randomized clinical study in subjects with metabolic syndrome, a phlebotomy of 500cc substantially reduced blood pressure and improved glycemic control in conjunction with the lipid profile (LDL / HDL radius), decreasing resting heart rate by 6 weeks: this noticeable improvement in blood pressure (and insulin sensitivity) it was directly related to the reduction in ferritin deposits (Housychar, 2012). Facchini et al. They were the first researchers to demonstrate in subjects with “healthy” insulin resistance that, after a single phlebotomy, hormone sensitivity and oral glucose tolerance are significantly improved, all of which is explained by the powerful relationship between Body iron deposits (serum ferritin) and insulin resistance (hyperinsulinemia) (Facchini, 1998).The reduction in blood viscosity and peripheral resistance are caused by blood collection, and conversely: approx. A 10% increase in Hematocrit produces a 20% increase in viscosity; all of which physiologically will lead to an elevation in blood pressure or excessive vasodilation. Then, only from a purely hemodynamic point of view, phlebotomy can induce an important antihypertensive effect, by reducing blood viscosity (Houschyar, 2012, rev). Molecularly, excess iron raises oxidative stress and determines an increase in vascular inflammation and the atherosclerotic process. Thus, iron chelator Dexferoxamine decreases the expression of adhesion molecules (reducing inflammatory stress) in animal models of local vascular inflammation: then, the capture of tissue iron emerges as an effective treatment in atherosclerotic vascular pathology by reducing mechanisms (related to irondependent toxicity) endothelial and vascular inflammation (Li, 2006).In an interesting study, Michalsen et al. they showed a dramatic improvement in arterial hypertension, glycemia and lipid profile after the removal of 550 cc of blood; and (apparently) independently of significant changes in insulin resistance, contrasting previous cross-sectional and cohort studies. This ambitious study demonstrates again the deleterious mechanisms of iron on vascular tone; rescuing an effective therapy (old) to combat a hypertension (Manco, 2012) resistant.In this context, the perfect action of insulin has anti-inflammatory and antithrombotic effects (Jara, 2003); it is considerably affected by the direct and indirect effects of excessive, free or accumulated iron (Fernández-Real, 2001). Accordingly, reductions in body iron are demonstrating - increasingly - improvinginsulin sensitivity (Facchini, 1998; Fernández-Real, 2005). It is extremely exciting to know today that the adequate activation in the Akt insulin signaling (serine-threonine-kinase enzyme) deeply preserves the cardiac function, and in accordance with the increased glucose uptake by the cardiomyocyte sarcolemma (Matsui. 2001).On the other hand, in relation to sudden cardiovascular death, we know that the prolongation of the QT interval is now considered a clear indicator of the increased risk of malignant ventricular arrhythmias and / or sudden death (Veglio, 2004). Thus, in particular, men with a prolonged corrected QT interval have an increased risk of sudden cardiovascular mortality; even in apparently healthy subjects (Shin, 2005).It is also known that prolongation of the QTc interval predicts mortality in diabetics. Faced with an unexpected prolongation of QT, she completely reverses with potassium infusions (Choy, 1997, Thomas, 2016), as we found in the Arrhythmias Intensive Cardiology Unit of the Hospital of Móstoles - Madrid; In addition to successfully treating Torsada de Pointes, maintaining a potassium in the upper physiological range (Thomas,2016) which improves the “ionic” Cellular Resistance to Insulin, by raising the kaliopenia or hypokalemia, acutely arrhythmogenic (Kjeldsen, 2010, rev).An altered metabolism is potentially arrhythmogenic, and today there is growing evidence that arrhythmias and metabolic activity are powerfully interdependent (Barth, 2013). Diabetes mellitus (type 1 and type 2) is a significant promoter of cardiac arrhythmias (Kannel, 1998; Grisanti, 2018, rev), especially Atrial Fibrillation, and independently (a 38 year follow up of Framingham heart study patients) (Benjamin, 1994). Moreover, a prediabetesinducing diet promotes arrhythmogenesis (Axelsen, 2015), and prediabetes, as epidemiologically proven, increases the risk of atrial fibrillation by 20% (Aune, 2018). Let’s not forget the clinical evidence that shows that arrhythmias are the leading cause of sudden cardiac death associated with transfusional iron overloads (thalassemia) (Siri-Angkul, 2018).On the other hand, regular donations, as summarized in a recent review by Holsworth, produce an obvious improvement in cardiovascular health, by significantly reducing total blood viscosity, plasma, hematocrit and fibrinogen, hemorrhaological parameters that damage rheology capillary and raise wall stress (shear stress); two pathophysiological parameters that powerfully influence the rupture and erosion of vulnerable atherosclerotic plaques (Holsworth, 2013), that is, those with the highest inflammation.Very interesting, it has been shown in vitro that soluble iron ions have been shown in vivo to increase the expression of genes related to cholesterol synthesis and fatty acid metabolism (Drinda, 2010).It is proven that, physiologically in “healthy” controls, the sharp increase in glucose can produce significant increases in the QT interval (and in the dispersion of the QTc) (Marfella, 2000); and this acquires greater relevance in critical patients, where hyperglycemia correlates with a longer duration of the corrected QT (Burkett, 2009). This, which naturally takes on greater gravity,Silent, in diabetic subjects, is recently confirmed by many studies: the QT interval is a predictor of total mortality in diabetic subjects or NO (Veglio, 2004). And this morbidity and mortality could be enhanced by the “western” overloads of exogenous iron. Consider the physiological increase of QT during sleep (Browne, 1983) due to the increase - similarly physiological - of Insulin Resistance during the early morning.A regulated restriction of labile iron (catalytic / free) continues to show positive effects on the quality of health in general, and on aging in particular (Gallaris, 2008). And, not to mention the obvious improvement in the action of insulin on the vessels and the endothelium that has the reduction of free iron, the idea of the current doctor is, as centuries ago, “first do no harm” with metals “physiologically” accumulated over time; All anti-inflammatory / antioxidant processes can operate effectively only in the absence of an excess of accumulated iron(Sullivan, 2004).Repeatedly it has been shown that only iron extraction or its restriction is capable of mobilizing and reducing macrophage iron from atherosclerotic plaques (Lee, 1999, Watt, 2006; Sullivan, 2007), thus stabilizing atheroma (Lee, 2003). In addition, in accordance with all of the above, we should point out the accumulated evidence: an increase in catalytic iron is compromised in the pathogenesis of renal disease: it has been shown that a ferric tissue overload stimulates angiotensin-induced renal fibrosis (Saito, 2004) On the contrary, chelation of iron reverses the stimulation of angiotensin-induced beta TGF in renal and cardiac tissue (Saito, 2004; Shah, 2009; Saito, 2005).On the other hand, if catecholamine oxidation can cause sudden death (Dalla, 2010, Adameova, 2009), and iron chelation is proven protective of its cardiotoxicity (Haskova, 2010), Emergency phlebotomy could prevent the imminence of Sudden death, as it has been shown, reduce the risk of heart attack (Salonen, 1998)? We believe with pathophysiological bases that yes.Let us not forget the reciprocal causal interrelation between Cardiovascular Autonomic Neuropathy and subclinical inflammation, (even in the presence of normal glucose tolerance in non-diabetics) (Vinik, 2012, Vinik, 2013, Herder, 2017); and that the direct parasympathetic and insulin-dependent antiinflammatory function is potentially aggravated by catalytic iron. We will use here to indicate with physiological evidence that a single phlebotomy (or blood donation) is able to stimulate erythropoiesis and improve chronic anemia (Kautz, 2014, a), through the new hormone Eritrofenone (which increases the release of sequestered ironby suppressing the production of Hepcidin (and physiologically increasing the increased formation of erythrocytes) (Kautz, 2014, b).Recently, phlebotomy has been shown to reduce systemic oxidative stress by decreasing fibrosis of the islets of the pancreas and increasing mRNA and antiinflammatory PPAR delta proteins (Minamiyama, 2010), which regulate cardiac reperfusion, increasing oxidation of fatty acids and the antioxidant enzyme system of cardiomyoblasts (Pesant, 2006, Minamiyama, 2010), thus inhibiting their apoptosis.In a recent meta-analysis, the highest risk of sudden cardiac death in the general diabetic and prediabetic population (Aune, 2018b; Aune, 2018c), with particular distribution in J in the Japanese population, where a low body mass index is found (under 19) confers greater long-term risk (Chei, 2008). The 82% higher relative risk of death (RR) occurs with increases of 0.1 in the Hip Waist index, compared with 16% of RR with 0.5 increases in the index (Aune, 2018c). That is, the relative risk of sudden long-term cardiovascular death is much higher in central or higher adiposity with overweight without obesity (than in those obese), which may be relevant in particular among thin men of Asian descent (Latin Americans). Therefore, blood donation among these people should NOT be restricted, and, quite the contrary, it should be stimulated, with special relevance for post-menopausal women with android fat accumulation, with increased cardiovascular risk (Chen GC, 2019).Today there is a cathartic evidence that shows that blood donation or extraction - and of course the restriction of iron in the diet) reverses the complications of diabetes, decreasing its current rapid progress (Ambachev, 2017, rev).Moreover, not only subjects with serum excess of free iron or ferritin should donate blood, but men with Hemochromatosis, given the discovery that they all present (to a greater degree than in Diabetes and Alzheimer’s Disease) a severe alteration in the morphology of the red blood cells and their membrane structure, causing their lower deformation and microvascular damage (extensively reviewed in: Pretorius, c, 2014).Today, it is also known that properly compensated diabetics have another enzymatic biochemical reason for their blood draw, since their erythrocytes can directly cause endothelial dysfunction (Zhou, 2018). Very interesting, it is the experimental and preclinical evidence that indicates that the lower concentration of leukocytes, until their depletion -granulocytopenia- prevents the pulmonary insufficiency caused, (Hetchman, 1983, rev), and is able to improve cardiac dysfunction due to lipotoxicity (Schilling , 2012). If it has been shown in diabetes that leukocytes (already increased in quantity) - massively release inflammatory cytokines, being macrophages of the M1 type (pro-inflammatory) (Bajpai, 2018, rev), and circulating monocytes secrete high amounts of TNF-x, reducing these myocardial perfusion (Wu, 2011); Regular donation or phlebotomy would be a magnificent therapeutic option, immediately reducing cytokine-induced inflammation promoting instability in ventricular repolarization (Sordillo, 2015, rev).
Insulin, Iron and Ischemic DiseaseWith particular importance in hypertensive subjects, several studies show that high hemoglobins, the greater oxygen release caused is closely related to the greater left ventricular mass, greater hypertrophy, and a deterioration in early diastolic filling: the greater hemoglobin, the greater the septal hypertrophy and of the posterior ventriculart wall (Narayan, 2006); thus enhancing the accumulated risk of sudden death, given that free hemoglobin or in contact with a broken atheroma plaque accelerates the process of atherosclerosis / prothrombosis and immediately (Potor, 2013, rev).Based on the myths of scientific folklore, countless iron-containing drugs continue to be prescribed as miraculous agents “givers of energy and vitality.” (Horwitz, 1999). Contrary to all this, cumulative evidence continues to demonstrate that excessive consumption, bioavailability and iron deposition can be extremely dangerous; even before the “normal” deposits of the metal, which over time, become excessive, due to their low elimination. Particularly, in a sedentary subject, consumption.
“Normal” iron - and its deposits as ferritin, can aggravate the disease in its presentation and prognosis (Fernández-Real, 2002; Weinberg, 2008; Brewer, 2007), by directly increasing the metal’s insulin resistance.Prediabetic subjects with evident and proven insulin resistance have a much more adverse cardiovascular profile than those individuals with less pancreatic capacity - that is, with a compromised function of beta cells (Haffner, 2000; Meigs, 2002). This implies that, the greater the amount of insulin generated (greater hyperinsulinemia / insulin resistance), the greater the cardiovascular risk (Reaven, 2012, rev). It has been shown that in subjects with evident insulin resistance, body accumulation in iron deposits exacerbates the action of insulin, aggravating endothelial dysfunction and cardiovascular function (Manco, 2012); and vice versa: iron depletion is very effective in improving endothelial and metabolic dysfunction, all of which is mainly due to a reduction in liver and systemic insulin resistance. In a representative sample of the American adult population, a high ferritin combined with a low transferrin saturation are associated, ofcompletely independent, at a higher risk of pre-diabetes (logistic regression analysis) (Cheung, 2013).The dangerous interrelationship between insulin, iron and ischemic heart disease is becoming increasingly important. The metabolism of iron, its contribution and the elimination of its accumulation, all this has begun to revolutionize the treatment, but above all the prevention of coronary heart disease. Thus, hepcidin, the hormone that regulates the tissue distribution of iron, preventing its excessive passage to the extracellular, would be increased early in acute myocardial infarction, and in its early phase (Suzuki, 2009). Moreover, its sharp increase promoted by iron causes an instability of atheroma - by mobilizing macrophage iron -, determining its greater inflammation (Li JJ, 2012) and the presentation or exacerbation of an unstable coronary artery disease. An optimum insulin sensitivity could protect against excessive accumulated iron?. Optimal insulin signaling - Akt serinethreonine kinase activation - preserves good cardiac function and prevents its ischemic injury (Matsui, 2001; Jara 2001, rev;Li, 2008), in response, to its experimental protection (Aikawa, 2000) and clinical (Sack, 2003) on the viability of the injured myocardium, especially for its powerful anti-inflammatory (Li, 2008; Jara, 2003) and antithrombotic effects (Jara, rev, 2003).This is clinically reaffirmed with the significant protection provided by the Insulin-Therapy unit in Intensive Care and ShockTrauma units (Sack, 2003; Teshima, 2010). If it is demonstrated experimentally and preclinically (Shamir, 2003; Teshima, 2010; Jara, 2001) that the optimal action of insulin is able to reduce atherosclerosis and prevent / protect myocardial cell apoptosis in heart attack (Aikawa, 2000); and all this in its preformed acute secretion - unlike the post-prandial insulin peak, nowadays inflammatory / atherogenic - (Jara, 2001, rev); the only obvious physiological and clinical fact that acute iron makes insulin resistant in its biological protective actionCardiovascular (Morales, 2000; Jara, 2001, rev), tells us how harmful the metal can be, when it accumulates more easily in the heart, because the iron in charge is the cause of apoptosis of HL-1 cardiomyocytes (Chen, 2014) , that is, in conditions of hemosiderosis (eg thalassemia or multiple transfusions), where it is deeply cardiotoxic and promoter of cardiomyopathy (Gammella, 2015, rev).Iron has a powerful immunomodulatory / regulatory function of vascular inflammation, by strongly promoting monocyte-endothelial adhesion in the development of coronary and systemic atherosclerosis (Kartikasari, 2009; Kartikasari, 2004). Moreover, it has been mentioned with pathophysiological and clinical bases that, iron, by itself can initiate the disease, in addition to promoting and aggravating it (Weinberg, 2008); all this, based on all the iron deposits observed in numerous local tissue processes - such as atherosclerosis; and vice versa. In many inflammatory and infectious conditions where macrophages are relatively iron deficient, there would be a host resistance to acquiring the infection, such as severe malaria.The problems that involve iron damage are especially important in the cardiovascular system. The existing chronic cardiac dysfunction (and cause of death) in the pathologies of excess in iron deposits has been known for decades. In a very interesting study, it has been reported that, even in patients with end-stage renal disease subject to hemodialysis, elevated iron deposits play a deleterious role in cardiovascular pathophysiology (Cheng, 2009). This corroborates, once again, the evidence: iron-mediated inflammatory processes cause tissue injury: chronic and acute; the latter by re-perfusion, proven in myocardial or cerebral infarction. Currently, the emerging evidence on the potential causal role of iron in the atherogenic process and in post-coronary angioplasty restenosis is accumulated (Horwitz, 1999).
In sum, the multiple benefits of blood donation or phlebotomy continue to be demonstrated for a long time in the powerful reduction of individual cardiovascular risk - in the short and long term - by the reduction of excessive “physiological” accumulated iron, high oxidative stress, inflammation and the potential prothrombosis to stress. (Holsworth, 2013; Day, 2003; Lipinsky, 2012; Pretorius, 2013). In view of the immense population that has a silent procoagulant state -deficient insulin action- (Reaven, 2012), the “normal” exogenous iron is potentially harmful, particularly at the cardiovascular level.The altered metabolism and distribution of iron are currently the most common pathophysiological condition in humans (Weinberg, 2008), and this, due to the “normal” presence of a low-grade inflammation existing in today’s man; which worsens with the physiological accumulation of body iron.We can state with much evidence that, the lower the iron in its normal range, the lower the organ-specific and systemic damage (acute insulin resistance); and greater the chance of survival in an accident”lethal”.
Left ventricular cardiac hypertrophy, with arrhythmogenic potential, can begin intrauterically by chronic glucose increase in the maternal-fetal placental unit: glucose elevation stimulates cardiac fibrosis induced by aldosterone in vivo (Sato, 1996); In particular, it is acute glycemic oscillations that promote cardiac fibrosis and the genesis of atrial arrhythmias in diabetes (Grisanti, 2018, rev, Ying, 2017, Saito, 2014). In addition, since reactive oxygen species facilitate apoptosis and cardiac fibrosis, glucose fluctuations are potentially aggravating arrhythmias, particularly atrial fibrillation (Dallak, 2008, Saito, 2014). Meta-analysis in clinical populations suggest a dose-dependent relationship between glycemia and atrial fibrillation; which implies that glucose levels are crucial for the onset of arrhythmia (Aune, 2018, Grisanti, 2018, rev).Remodeling (hypertrophy) and cardiac fibrosis could be enhanced by excess iron or its acute contribution, which further alters glucose metabolism (Fernández-Real, 2002, rev); It is she who finally regulates the concentration of serum iron (Aigner, 2013). This arrhythmogenesis has a higher lethality at dawn in coronary subjects, since there is a circadian variation of myocardial ischemia due to an increase in ST depression (Rocco, 1987); coinciding with a physiological nocturnal increase in insulin resistance.Finally, it has been shown that lack of sleep (deep sleep disruption) promotes the excessive formation of immature monocytes (by reduction of the hypocretin hormone), increasing vascular inflammation and atherosclerosis (McAlpine, 2019), we affirm categorically, need to donate blood in any subject that does not sleep well, and in particular, if he has suffered a traumatic brain injury (Daglas, 2018, rev). Even in subjects who have suffered a myocardial infarction, themassive leukocyte influx into ischemic tissue, is extremely inflammatory (Schilling, 2012, Anzai, 2017), and could immediately benefit from a phlebotomy - a reduction of these inflammatory cells - (Schilling, 2012), as well as patients with ovarian polycystosis (hidden insulin resistant), those with excess ferritin, hemoglobin and leukocytes (which can be reversed with aerobic exercise) (Covington, 2016). An atherogenic inflammatory role of platelets has just been demonstrated - and in the absence of thrombosis- (Barret, 2019).
1- Current medicine is a Dynamic Science: thus, as a patient cannot be ruled out of an acute coronary syndrome due to a negative coronary angiography (which does not “see” the instability / inflammation of the atheroma), “healthy” subjects should be warned if thoroughly evaluated before a sudden exercise: acute hypertensive changes that can be fatal (together with electrolyte changes / EKG) can be predicted with the previous degree of insulin resistance (Brett, 2000) and body ferritin. Let us not forget that it is the neuroendocrine system and its response to acute stress, which establishes morbidity and determines Mortality in the patient with any acute coronary syndrome (Pérez de la Hoz, 2018, rev).
2- Normotensive subjects with abdominal obesity present with unusual frequency a cardiac remodeling due to ventricular fibrosis associated with an obvious diastolic dysfunction (Eschalier, 2014, Cavalera, 2014, rev). It has been reported in non-diabetic and non-overweight normotensive individuals (with central fat accumulation) ventricular concentric hypertrophy and diastolic dysfunction (Eschalier, 2014). And the diastolic dysfunction will be greater the greater the myocardial resistance to insulin, since the synthesis and replacement of intracardiac collagen is evident and in direct relation to Insulin resistance (Quilliot, 2005, Cavalera, 2014, rev). Since insulin resistance profoundly alters the turnover of cardiac collagen, increasing its production, its strong and independent contribution to myocardial fibrosis (in the absence of hypertension) has been recently demonstrated (Quilliot, 2005).
3- Unlike chronic hyperinsulinemia, acute exogenous insulin (or preformed insulin, in the presence of good tissue sensitivity) has anti-atherogenic protective effects, which are canceled in any state of Insulin resistance (Jara, 2001, Hsueh, 1999, Low-Wang, 2003): Normophysiological acute insulin by itself, in the absence of endothelial dysfunction or excess iron, maintains the phenotype and quiescence of the vascular muscle cell; and its sensitivity is decisive to prevent or generate coronary vasospasm in nondiabetics (Kang, 2018).
4- We can confirm that, in accordance with a recent Japanese study among low-risk population (JACC Study), high iron intake is positively associated with higher mortality from global cardiovascular disease (and total stroke mortality) in men (Zhang , 2012), where iron accumulates physiologically with progressive rapidity (Arruda, 2013). Solid experimental and epidemiological evidence demonstrates the direct relationship between high iron deposits and the increased risk of cardiovascular disease, prediabetes and diabetes (Basuli, 2014).
5- Excessive levels of reactive oxygen species and accumulated iron, both due to iron overloads induce mitochondrial dysfunction, reducing the energy levels of the cell and raising gluconeogenesis (Lee, 2014; Lee HJ, 2015): This explains the powerful generation or exacerbation of insulin resistance in particular predisposing to coronary pathology (McLaughlin, 2004, Reaven, 2011; Reaven, 2012) -
6 - A high intake of iron (heme) would increase the risk of suffering a fatal myocardial infarction, especially given a low intake of dietary micronutrients (Kaluza, 2014), such as flavonoids and catechins, fruit “chelators” of the metal. Iron by itself canaffect macrophage plasticity, and its excess would promote destabilization of atheromatous plaque by powerfully increasing its inflammation (Boyle, 2009, Vinchi, 2014, Saeed, 2012). Its high consumption should be banned in populations with high cardiovascular risk, since it increases in these populations the risk of suffering from Type 2 Diabetes (Fernández-Cao, 2013). The functional and structural damage to the red blood cells of the diabetic - caused by iron - is reversible with blood collection (Pretorius, 2014, Buys, 2013).
7- In addition to its multiple vascular actions on cardiac cells, insulin regulates cardiac electrophysiology, and decreasing its action (signaling) alters ventricular electrical remodeling, increasing the risk of sudden death; Thus, insulin resistance can be (and is) the primary cause of cardiovascular disease (Dubó, 2014, rev) in prediabetes and occult diabetes.
8- Everything shown here, and contrary to what is traditionally held, proves once again that it is the inflammatory, particularly cytokine-induced environment that determines the genesis and complications of cardiac pathology (Jara, 2003, Demir, 2016). And it is the acute inflammation of the atheroma plaque that is promoted by the mobilization of tissue (hormone-dependent) iron (Li, JJ, 2012), which would initiate the acute ischemic episode. Even, the vascular endothelium is rapidly altered in response to low doses of iron, which also damages DNA (Mollet, 2016). And iron in charge generates mitochondrial dysfunction (Lee HJ, 2015), and at physiological doses it increases adipocyte inflammation in the entire insulin-resistant population (Chirumbolo, 2015), stimulates hepatic gluconeogenesis, being molecularly, diabetogenic (Simcox, 2015). Thus iron is a proven direct causal factor in the pathogenesis of diabetes (Simcox, 2013, rev, Ashok, 2019).
9- It is strongly demonstrated that the prolongation of the interval QT contributes and can PREDICT cardiovascular mortality in “healthy” people (Schouten, 1991, Marfella, 2001), which can be sudden in “normal” men with insulin resistance (Munger, 1991, Kurl, 2016, Piccirillo, 2007, Kahn, 1996); and especially before endogenous or exogenous iron overloads (Rose, 2011). It has recently been shown that an obesity-inducing diet causes a lengthening of QT and increases ventricular ectopia in obese rodents (Huang, 2013).
10- The Function Determines the Structure (Mather, 2004, rev). Recall that a metabolic phenomenon causes a microvascular phenomenon and vice versa: early insulin resistance is the earliest event and that precedes muscle metabolic insulin resistance (Zhao, 2015).
11- In the Spanish market there are more than 100 drugs capable of prolonging the QT interval, (Jiménez-Ortega, 2006), by altering the channels and reducing the body’s potassium levels (Calderone, 2002, rev, Sordillo, 2015, rev, Mladenka, 2018) We alert the Peruvian Scientific Community of the serious danger of dispensing many of them without prescription (several antihistamines, neuroleptics or antibiotics such as clarithromycin). Likewise, any iron supplement - or added to food - today becomes potentially harmful, especially for the coronary system (Kang, 2001, rev; Chirumbolo, 2015).
12- If every subject reduces their insulin resistance, they will be able to rejuvenate molecularly - Shortening of telomeres in leukocytes is largely due to Insulin Resistance - (Demissie, 2006). Although, in a life without stress (environmental-nutritionalmetabolic-hormonal) intracellular iron is not toxic (Eid, 2017) - which is currently impossible - its “normal” daily exogenous consumption programs cell death or Neoplastic proliferation, depending on our degree of serum insulin. And if iron overload cardiomyopathy is on the rise today (Gujja, 2010), it is expected that coronary iron heart disease, too.
13- Let’s not forget that the capture of tissue free iron can save a life, from a cerebral hemorrhage to a sepsis (Kazmierski, 1996, Hanafy, 2012, rev); since its greater tissue bioavailability will determine the degree and severity of organic injury (McCullough, 2011, rev). The aggravation of sepsis due to iron therapy and the increase in its mortality due to profound iron-induced cardio.renal damage is due to the profound potentiation of systemic inflammation - exacerbation of the lipopolysaccharide inflammatory response - in vitro and in vivo (Zager, 2004, rev; Hoeft, 2017).
14- Given the molecular evidence that proves that the hormonal metabolic environment regulates the expression of genes in the heart (Young, 2007, Ballal, 2010), and that metabolic disorders precede, generate and aggravate cardiac pathology (Wang, 2004, rev, Saotome, 2019, rev, Sung, 2019, Sundell, 2003, Muniyappa, 2007, rev) it is time to replace the term “Cardiovascular Pathology” with Cardiovascular Metabolic Pathology (Cardiovascular Endocrinology) (Wang, 2004, rev, Ballal, 2010 , Sung, 2019). And the risks for all this arterial pathology (vascular resistance to insulin) -programmed intrauterine- acquire a greater presence in the presence of prenatal hypoxia (Walton, 2016, Chen, 2019) throughout Latin America.
15- Iron plays a causal role in the development and progression of atherosclerosis and coronary heart disease (Vinchi, 2014, rev, Sullivan, 2007, rev, Xu S, 2019a), given that its overload is atherogenic, and its depletion by phlebotomies or dietary restriction reduces vascular inflammatory mechanisms, reducing their progression, and being able to reverse them (Xu, 2019b, rev; Vinchi,2019), as has been shown, recently in animals and humans (Vinchi, 2019), after the publication of this article.After the publication of this article, it is recognized in the journal Nature, that Cardiovascular Pathology belongs to the wide range of Cardiometabolic Diseases, together with ischemic brain pathology and Diabetes (Miranda, 2019, rev).
