Author(s): Rogean Rodrigues Nunes, Filipe Mitsuo Akamine*, Bruno Rocha Meireles, Daniel Gomes de Moraes Nobre and Jose Carlos Rodrigues Nascimento
Objective: To evaluate the influence of low doses of S-ketamine, during total intravenous anesthesia, on the suppression rate (SR) induced during total intravenous anesthesia (TIVA) by deepening the anesthetic plane.
Methods: A triple-blind clinical trial was conducted with 20 patients who underwent elective operations under general anesthesia, randomly allocated into two groups, CG (control: 0.9% saline) and CGS (s-ketamine: 0.2 mg.kg-1). Each group was assessed at M0 (immediately before pre-oxygenation), M1 (3 minutes after SR stabilization) and M2 (3 minutes after infusion of s-ketamine or 0.9% saline). Bispectral index (BIS), electromyography, and SR were recorded at each moment.
Results: There were no statistically significant changes regarding BIS and EMG from M1 to M2. There was also no indication of a significant difference between the average SR values concerning the factors group and moment, jointly (p=0.499), and separately (G1 and G2, p=0.812 and p=0.923).
Conclusions: Low doses of s-ketamine were insufficient to determine changes in SR values, validating this important electroencephalographic parameter.
Significance: The study findings reinforce that low doses of s-ketamine do not invalidate the SR values.
S-ketamine is used as an adjuvant drug during general anesthesia because of its important analgesic and hypnotic properties, thus reducing the need for opioids and hypnotics [1]. However, several studies have shown important elevations in electroencephalographic activity caused by this drug. The bispectral index (BIS) is a parameter derived from the electroencephalogram (EEG) analysis, resulting from the analysis of three sub parameters: burst-suppression, fast Fourier transform, and bispectrum [2]. S-ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonists commonly used in general anesthesia [3]. It presents a pattern recognizable to the spectrogram, with the presence of gamma burst in the generation of unconsciousness, an increase of slow delta frequencies (0.1 - 4 Hz), theta (4 - 8 Hz) and gamma (27 - 40 Hz) oscillations, and reduction of alpha/beta frequencies (10 - 24 Hz) [4, 5]. This study aims to evaluate the influence of low doses of S-ketamine, during total intravenous anesthesia, on the suppression rate (SR) induced during total intravenous anesthesia (TIVA) by deepening the anesthetic plane.
A triple-blind clinical trial was conducted after submission to Plataforma Brasil (the official research launching system of Brazil) and approval by a Clinical Research Ethics Committee (CAAE: 61250022.3.0000.5043). All participants signed an informed consent form.
We evaluated 20 patients, of both sexes, who underwent elective operations under general anesthesia, with a physical status P1 (American Society of Anesthesiologists), aged between 20 and 50 years, and with a body mass index between 21 and 26 kg.m-2. Patients were randomly allocated into two groups: CG (control) and SKG (S-ketamine), with 10 participants in each group, using a restricted (sequential) randomization method. CG - Bolus of 0.9% saline, IV, in a volume of 10 mL. SKG - 0.2 mg.kg-1 of S-ketamine, IV, prepared to a volume of 10 mL in 0.9% saline. In both groups, the solutions were infused within 60 seconds.
Patients that recently used drugs that could alter the electroencephalogram were excluded.
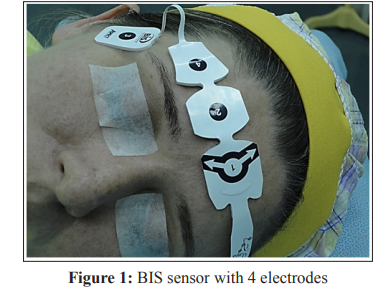
1. BIS Vista° monitoring system, version 3.5, with bispectral
index (BIS), suppression rate (SR), and electromyography
(EMG) readings. The electrodes were adapted at the following
points FPz (referential-electrode 1), FP2 (virtual groundelectrode 2), F8 (electrode 4), and FT10 (electrode 3) -
(Figure1).
2. Anesthetic gas analyzer;
3. Electrocardiogram in two channels (DII and V5);
4. Pulse oximeter;
5. Capnography monitor;
6. Automatic non-invasive blood pressure monitoring system;
7. Convective forced thermal air heater;
8. Infusion pump with effector site (ES) programming, using the
following pharmacokinetic models: Minto, for remifentanil,
and Schnider, for propofol;
9. Thermometer with a nasopharyngeal sensor
All patients were previously submitted to preoperative clinical and laboratory assessments.
None of the patients received pre-anesthetic medication. All were submitted to the effects of the same anesthetic technique. After venipuncture in the right upper limb with a 20G catheter, Ringers lactate solution (RL), 2 mL.kg-1 was administered to all patients for fasting replacement and 4 mL.kg-1 to replace intraoperative losses. In both groups, pre-oxygenation with 80% oxygen was performed using a mask, 5 minutes before drug infusions until immediately before orotracheal intubation. Anesthetic induction was performed with sufentanil 0.3 μg.kg-1, intravenously, with simultaneous infusions of propofol, controlled effector site (ES), with an initial target of 3.0 μg.mL-1 and remifentanil hydrochloride (ES), with an initial target of 3 ng.mL-1 until the BIS value reached 40. If the BIS did not reach 40, the ES concentration of remifentanil was increased by 0.5 μg.mL-1 in 0.5 μg.mL-1 until a BIS of 40 was obtained. At this moment, the concentration of remifentanil hydrochloride was fixed, and orotracheal intubation was performed. After the intubation, the respiratory rate was adjusted to maintain PETCO2 between 35 mmHg and 40 mmHg, with a FiO2 of 35% and a tidal volume of 8 mL.kg-1. Ventilation -1 was carried out in a circular system with a CO2 absorber. Propofol and remifentanil infusions were adjusted to maintain a BIS in the closed range of 45 to 60, following the guidelines shown in Figure 2 [5]. Next, modifications in propofol concentrations were performed 0.5 by 0.5 μg.mL-1 with subsequent variations performed 2 minutes after reaching the concentration at the ES, aiming to determine a suppression rate between 10% and 20%. A neuromuscular blocker was not used due to the possibility of interference in the EEG readings [6].

For clinical purposes and statistical evaluations, 3 moments were
analyzed in each group:
1. M0 - Immediately before pre-oxygenation.
2. M1 - 3 minutes after SR stabilization between 10% and 20%.
3. M2 - 3 minutes after infusion of s-ketamine or 0.9% saline.
The following variables were recorded at each moment: BIS,
Electromyography (EMG), and SR. The protocols hemodynamic
variables considered lower limits were SBP=80mmHg and
DBP=50mmHg. The upper limits for SBP and DBP were
clinically significant if they exceeded 20% of baseline (verification
performed upon admission to the operating room). Heart rates
with variations greater than ±25% of baseline (before anesthetic
induction) were considered clinical significant. In all patients, the
nasopharyngeal temperature was maintained between 35 and 36°C
with a thermal sheet of forced convective hot air. Patients whose
hemodynamic variables exceeded pre-established values in at
least one of the evaluated moments were excluded from the study
The data obtained were compared both between moments in the same group and between groups at equivalent moments.
The sample size calculation was performed considering an α=5% error, β=20% error, a 3% difference to be detected, a standard deviation of 2, and a two-tailed hypothesis test, which resulted in 7 patients for each group. Descriptive statistics, graphics, and analysis of variance with repeated measures were used in the statistical analysis, and P values≤0.05 were considered significant. The statistical analysis was performed using SPSS version 26.0.
The two groups were considered homogeneous regarding age, weight, physical status, and height. Regarding BIS and EMG, there were no statistically significant changes comparing M1 andM2. Through the analysis of variance (M1 and M2), it was verified that there was no indication of a significant difference between the average SR values concerning the factors group and moment, both when considered jointly (p=0.499), and separately, G1 and G2 (p=0.812 and p=0.923, respectively, Table 1 and Figure 3).

S-ketamine, due to its analgesic and hypnotic properties, has been used frequently during general anesthesia. In addition, it has several beneficial effects, including neuroprotection, preemptive analgesia, preventive analgesia, and reduction of opioid-induced tolerance and hyperalgesia [7]. Low-dose ketamine is defined as a bolus dose of less than 2 mg.kg-1, administered intramuscularly and less than 1 mg.kg-1, administered intravenously or epidurally. For continuous intravenous administration, low-dose ketamine is defined as an infusion rate of less than or equal to 20 μg.kg-1 [8, 9].
In a randomized, blind study, low doses of s-ketamine (100 μg.kg-1), in bolus, followed by continuous infusion of 2 μg.kg-1. min-1during radical prostatectomies reduced, in the first 48 hours after the operation, the consumption of morphine by 34% and decreased the intensity of pain at rest compared to the control group, which did not receive s-ketamine [10]. Many studies show that small doses of ketamine, in boluses, followed by continuous infusion, can provide better control of both the anesthetic act and postoperative pain without showing adverse psychic effects in the postoperative period, nausea, vomiting, or sedation [8-10].
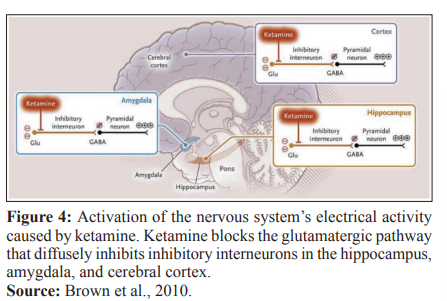
The processed electroencephalographic analysis is an important method for monitoring anesthetic depth and shows a good correlation with several agents used in clinical practice including propofol, midazolam, isoflurane, and alfentanil [11]. Unlike unconsciousness induced by various anesthetic agents, predominantly associated with EEG slowing, ketamine-induced unconsciousness is associated with EEG activation. Ketamine preferentially inhibits glutamatergic impulses to GABAergic interneurons, resulting in aberrant excitatory activity in the cortex, hippocampus and limbic system, causing unconsciousness [12] - Figure 4.
The hallucinations observed with ketamine may result from this aberrant activation that allows the association of information inconsistently in time and space. These hallucinations can be mitigated by drugs that act on or elevate the activity of GABAA-mediated interneurons [12]. Ketamine alone does not reduce BIS values even when patients are unconscious. Hirota et al. [13]. Showed that the administration of ketamine at a dose of 0.4 mg.kg-1, followed by an infusion of 1 mg.kg-1.H-1, increased the BIS from 44.1±0.7 to 58.6±1.4 (p<0.01), with significant increases in the SEF 95% being observed. A study by Dahaba showed that ketamine does not follow the pattern observed with other anesthetic agents during induction, which increases potencies in lower frequency bands [14]. On the contrary, ketamine increases the BIS value, simultaneously reducing delta potencies and increasing beta potencies, and simulating awakening
Carrara et al. compared the administration of s-ketamine in bolus 0.25 mg.kg versus continuous infusion of 0.25 mg.kg.h-1 at the time of surgical incision, and an increase in BIS values were observed in the first 15 minutes, with a significant difference between groups (p < 0.0001) indicating adequate monitoring of anesthetic depth with continuous infusion [15]. HambrechtWiedbusch et al. compared subanesthetic doses of s-ketamine (25 mg.kg-1 via intraperitoneal route) in rats under general anesthesia with isoflurane (bolus administration 90 minutes before the end of surgery versus administration of saline solution in the control group) [16].
There was a higher incidence of SR and a sign of great anesthetic depth in animals that received s-ketamine. However, a paradoxical reduction in awakening time was observed after isoflurane withdrawal, with increased cholinergic activity in the prefrontal cortex and cortical activation.
Sengupta et al. compared ketamine doses of 0.2 mg. kg-1 with 0.5 mg.kg-1 and the results showed that only doses of 0.5 mg. kg-1 significantly raised the BIS. Nonaka et al. investigated the effects of ketamine on BIS during general anesthesia (BIS maintained between 35 and 45, before ketamine), with target-controlled infusions of propofol and remifentanil, comparing two groups: the first received 0.2 mg.kg-1 and the second 0.5 mg.kg-1, in addition to the control group, which did not receive ketamine [17, 18]. The authors concluded that only the group with higher doses of ketamine significantly altered the BIS (p<0.05). A recent study using s-ketamine, 0.2 mg.kg-1 IV bolus, followed by continuous infusions of 100 μg.kg-1 IV, 200 μg.kg-1 IV, 300 μg.kg-1 IV or 400 μg.kg-1 IV, showed no significant changes in BIS during total intravenous anesthesia with propofol and remifentanil [19].
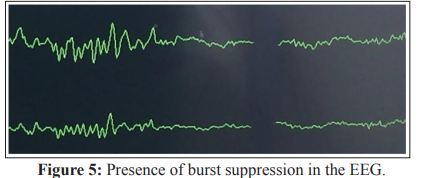
No human studies were found in the literature evaluating low doses of s-ketamine during controlled total IV anesthesia and its influence on SR. The SR is an important variable in the time domain, available in BIS monitoring, and represents the relationship between the time in which the EEG shows suppression (voltage less than ±5μV for a time greater than 0.5s), over the last 60s (30 epochs), with a value equal to zero being considered normal - Figure 5 [2].
Suppression rate is a variable of great independent value and is associated with three situations: cell ischemia, hypothermia, or deep anesthesia [7, 12]. Watson et al. demonstrated that non-zero suppression is associated with increased mortality [20, 21].
Puri et al. concluded, in a study performed during cardiac surgery, that SR values can be used as early indicators of brain dysfunction. Fernandez Suares et al. in a recent publication, showed that, after technical problems with the cardiopulmonary bypass equipment, the SR increased from 0 to 100, returning to normal values (zero) after reestablishing the flow [22]. Widespread burst suppression after cardiac surgery is generally associated with a poor neurological prognosis [23]. The term burst-suppression indicates bursts of the electrical activity of varying duration interspersed with periods of generalized suppression and seem to be related to the functional dissociation between the cerebral cortex and the thalamus [24].
S-ketamine was related to increases in electrical activity in the nervous system. Suppression rate could be reduced, resulting in unreliable values during episodes of cerebral hypoperfusion. In this study, low doses of s-ketamine were insufficient to determine changes in SR values, validating this important electroencephalographic parameter. It is concluded that low doses of s-ketamine (0.2 mg.kg-1) in our sample of patients under total venous anesthesia did not invalidate the SR values observed during anesthetic monitoring.
