Author(s): <p>Khulood Abdulrazaq Kaleel<sup>*</sup>, Wijdan Nazar Ibraheim and Qussay Nfawa Thaqab Almaliki</p>
ASD are particularly heterogeneous developmental conditions. Many arguments clarified a central function for dysregulation of immune system in ASD and many ASD hazard genes encode the immune system elements along with other risk factors. There is also evidence of continuing dysregulation of immune system in people with ASD and in animal models of this condition.
Objectives: This study was performed to investigate the relationship between the IFN-γ + 874 gene polymorphism and INF - γ plasma level with susceptibility to ASD
Methods: Polymorphism detection analysis was performed on 194 subjects from Basra south Iraq. 94 patients with ASD, (78 (83%) males and 16 (17%) females), their age ranging from 2 to 13 years, and 100 children apparently healthy control group matching for the same age and sex The IFN-γ gene polymorphism (+874A/T) was genotyped through a specific sequence primer polymerase chain reaction (SSP-PCR). For all participant the plasma level of INF-γ was determined by ELISA KIT (Elabscience, USA, Catalog No: E-EL-H0108), followed the manufacturer instructions
Results: showed sufficient differences in the plasma concentration of INF between children with autism and the none group: 24.87 pg/mm2 18.86 pg/mm respectively, with significant increase in plasma concentration of IFN of male and female of autistic group as compare to control group. Results of iterative distribution of INF-γ+874 gene polymorphism showed heterogeneous results between autistic group and control group, as the T allele was scored in autistic group 23.4% in compared with A allele which scored 79.7%, while in control group the T allele was scored 67% in compared with A allele which was scored 85%. AA genotypes of IFN-γ+874 showed higher recurrence among autistic group in compared to control group,(76.5% vs 33% respectively), while TA genotypes of IFN-γ+874 showed lower recurrence in autistic group when compared to control group,(3.2% vs 52% respectively),and IFN-γ+874 TT genotypes scored 20.2% in autistic patients and 15% was scored in control group ASD susceptibility is associated with the T allele of +874 rs2430561 with increase plasma level of IFN-γ which may have role in the severity of behavioural changes.
Autism spectrum disorder (ASD) is estimated a heterogeneous and complex neurological condition. It is relized by the Diagnostic and Treatment Manual for Mental Disorders, Fifth Edition (DSM5) and is differentiated by limited-repeated kinds of activities, behaviour, or interests, and persistent chronic deficiencies in connection with social contact ASD etiology has long been a widely debated topic and stills weakly understood. Double studies supply proof that ASD susceptibility may have genetic, heritability, in addition to significant environmental components. A strong inflammatory state associated with ASD reported increasingly in the resent years. The inflammatory disorder is also associated with dysfunction of the immune system [1-8]. In ASD children enhanced inflammatory activity has been indicated through analysis of biomarkers of pro-inflammatory. Since central neurotransmitters are closely associated with cytokines and stress is influence in their regulation perhaps most autistic peoples exhibit immune abnormalities, including increased level of IL-1, IFN-ɣ and TNF-α in the plasma and/or CSF. One of a pro-inflammatory cytokine is an interferon gamma (IFN-ɣ ) and an engaging possibility for a capability to psychosis. IFN-ɣ is essentially generated by (NK) cells and Th1lymphocytes, there is substantiation that IFN-ɣ and its receptor are generated by glial cells and neurons within the nervous system. IFN-ɣ precedes homeostatic role under physiological situations by effective immediately on neurons of CNS in order to control communication of neuron and public ability [9-14].
A single gene is responsible for IFN-ɣ production which planned on the chromosome 12 (12q15). Also, associated single nucleotide polymorphism (SNP) A to T switch at +874 position (rs2430561) is repetitively linked with raised serum cytokine and concentration of IFN-ɣ mRNA. Three possible genotypes are produced by analysing the sequence mutation for the genotypes A/A, T/A and T/T of + 874 IFN-ɣ (whether T or A), which confer three variant phenotypes0: weak, intermediate and high IFNG producers respectively. Recent studies of genetic association have supplied substantiation for disease-associated differentiation in genes of the natural and acquired immune system [15-18].
This study was conducted to investigate the association of the IFN-ɣ gene polymorphism with its plasma level in ASD patients.
This is a case - control study in which Autistic children (patients group) and apparently healthy control individual (control group) were enrolled from the same population living in Basra providence in Iraq.
A total of 94 patients with ASD, (78 (83%) males and 16 (17%) females), their age ranging from 2 to 13 years, were registered in the study who have been diagnosed by specialist in psychiatry, Dr. Qussays Private Clinic in Basra, Iraq
A total of 100 (80 male and 20 female) apparently healthy children, who were attending Primary Health Care Centers (PHC) (in the center and peripheral areas), for vaccination with no history of autistic spectrum disorder in family. The control group was matched with the patients group by their age and gender.
This study received approval by the Primary Health Care Centers manager and family of both patients and control kids. Its procedures and purpose were clarified to all studied population, and every individual member of the family has obtained written informed consent.
Three mL of venous blood was drawn from each Participants using a sterile disposable syringe and divided as following: 1 ml of the sample was emptied into an anticoagulant EDTA tube (Ethylene Diamine Tetra Acetic acid) which used in whole DNA extraction to detected INF gene polymorphism, and remaining blood emptied in other EDTA tube, last tube was centrifuged at 3000 rpm for 10 mints and plasma transferred to sterile plane tube to use in ELISA technique.
Plasma levels of INF were evaluated using an ELISA (Elabscience, USA, Catalog No: E-EL-H0108), according to manufacture protocol.
From patients and control samples, Whole DNA was extracted by using DNA Blood Mini Kit (Favorgen -Europe Cat. No: HB10.03.10 UK) depend on kit manufacture supplied with kit, the DNA extraction stored at -20 until use.
Genotyping of IFN- ɣ +874 polymorphisms was done by the single Nucleotide polymorphism SNP-PCR method. The polymorphic site in IFN-ɣ +874 gene contained 241 bp fragment was amplified using PCR primers listed in table (1) the mixture of reaction which used for observation of IFN-ɣ +874 gene polymorphism was in a total volume of 25𝓊 l including antisense primer 1𝓊 l/100 pmol/𝓊 l, specific A primer 1𝓊 l/100 pmol/𝓊 l, or specific T primer 1𝓊 l/100 pmol/𝓊 l, green master (USA) 12.5𝓊 l, sterile Nuclease free water 8.5𝓊 l and genomic DNA 2𝓊 l. The protocol of PCR was done in a thermocycler which including: 95° C(3min), the cycling: 10 cycles of 95° C (15 s), 65° C (50s) and 72° C (40s), followed by 20cycles of 95° C (20s), 55° C (50s) and 72° C (50s) and a final extension 72° C (7min). Products of amplification were visualized on an ultraviolet transilluminator after displayed to 2.5% agarose gel electrophoresis, stained with ethidium bromide.
The Statistical Package of Social Science (SPSS, version 20) were utilized to examine and processed data. The IFN-ɣ allele frequencies were analyzed by the Hardy Weinberg equilibrium for autistic and control groups using the 𝒳 2 test. Also analysis the important link between ASD (autistic against controls) and genotypes of IFN-ɣ . The connection btween polymorphism of IFN-ɣ with its level was analyzed by utilizing 𝒳 2 test, and IFN-ɣ concentration was tested for autistic and control group utilizing the T-test. The two-tailed study tested the gaps between the groups for significance. The p value of <0.05 was considered significant.
Table 1: Oligonucleotide Primers Sequences Used for PCR Amplification of INF-ɣ Gene Polymorphism [19].| Primer Name | Sequence (5-3) |
|---|---|
| Specific A | 5`-TTCTTACAACACAAAATCAAATCA-3` |
| Specific T | 5`-TTCTTACAACACAAAATCAAATCT-3` |
| Antisense | 5`-TCAACAAAGCTGATACTCCA-3` |
Results showed sufficient differences in the plasma concentration of INF between children with autism and the none group, undergo the probability level 0.05. As the probability value has reached 0.014, as in table (2) which indicated a marked increase in the mean concentration of INF in the plasma of the autistic children in compared to control group which were 24.87 pg/mm2 18.86 pg/mm respectively
Table 2: Concentration Level of IFN-ɣ in Population Study| Test | Autistic group No.=94 | Control group. No. =100 | P value |
|---|---|---|---|
| mean ± SD | mean ± SD | ||
| IFN-ɣ | 24.87 ± 2.003 | 18.86 ± 1.38 | 0.014 |
Table (3) was indicated significant differences in plasma concentration of IFN between male of autistic group and male of control group undergo probability value 0.05. As the probability value reach 0.05 which was cleared marked increased in IFN concentration in male of autistic group 24.2 while in male of control group IFN concentration reached 19.1. Also the same table showed significant differences in IFN concentration in plasma between female of autistic group and female of control group. As the p value reach 0.04 which was seemed marked increased in IFN concentration in female of autistic group 27.1 while in female of control group IFN concentration reached 14.8.
Table 3: IFN-ɣ Concentration Level among Sex| Studies Group | IFN-ɣ Concentration | Total | |||
|---|---|---|---|---|---|
| Male | Female | ||||
| n=. | Mean± SD | n= | Mean± SD | ||
| Autistic group | 78 | 24.2 ± 18.7 | 16 | 27.1 ± 23.3 | 94 |
| Control group | 81 | 19.1 ± 14.7 | 19 | 14.8 ± 8.5 | 100 |
| P value | 0.05 | 0.04 | |||
The role of sex of ASD on the level of plasma IFN-ɣ was come into view in table (4), the results showed no significant differences in IFN-ɣ concentration in plasma between male and female of autistic group undergo probability value 0.05.
Table 4: IFN-ɣ Concentration Level in Sex of Autistic Group| Variable | Autistic group No. (94) | |
|---|---|---|
| Male No. (78 ) | Female No. (16) | |
| Mean ± SD | Mean ± SD | |
| IFN-ɣ | 24.40 ± 18.6 | 27.1 ± 23.3 |
| P value | 0.6 | |
The results of the electrophoresis of IFN-ɣ gene polymorphism amplification T/A+874 were shown two alleles T/A and 3 genotyping (TT, TA, AA). Results of iterative distribution of IFN-ɣ +874 gene polymorphism showed heterogeneous results between autistic group and control group, as the T allele was scored in autistic group 23.4% in compared with A allele which scored 79.7%, while in control group the T allele was scored 67% in compared with A allele which was scored 85% figure 1. Also table 6 showed significant differences in repetitive distribution of T allele between autistic group and control group, the results were indicted that T allele was showed significant repetitive in low percentage in autistic group from control group. While A allele with no significance relationship.
IFN-ɣ level in plasma with IFN-ɣ +874 genotypes showed highly significant differences between autistic group and control group undergo probability value 0.05 table 6.
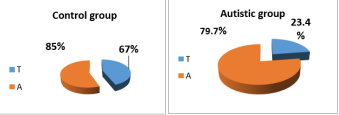
Figure 1: IFN-ɣ Alleles
Table 5: Frequency of IFN-ɣ Gamma Allele in Studies Group| Allele | Autistic group | Control group | X2 | df | P value |
|---|---|---|---|---|---|
| T | 22 (23.4 %) | 67 (67 %) | 37.0 | 1 | 0.000 |
| A | 75 (79.7 %) | 85 (85 %) | 2.04 | 2 | 0.35 |
AA genotypes of IFN-ɣ +874 showed higher recurrence among autistic group in com-pared to control group, (76.5% vs 33% respectively), while TA genotypes of IFN-ɣ +874 showed lower recurrence in autistic group when compared to control group,(3.2% vs 52% respectively), and IFN-ɣ +874 TT genotypes scored 20.2% in autistic patients and 15% was scored in control group. Figure 2
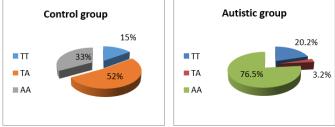
Figure 2: IFN-ɣ Genotypes
Table 6: Correlation between IFN-ɣ Genotypes Plasma Level and IFN-ɣ Genotyping Among The Study Group| TT | TA | AA | ||||
|---|---|---|---|---|---|---|
| Group | Frequency | IFN-ɣ con. mean | Frequency | IFN-ɣ con. Mean | Frequency | INF con. mean |
| Autistic group | 19 | 26.71 | 3 | 19.66 | 72 | 24.61 |
| Control group | 15 | 16.30 | 52 | 11.04 | 33 | 21.46 |
| P. value | 0.00 | 0.00 | 0.00 | |||
Table 7 was showed distribution of IFN-ɣ +874 T/A alleles and IFN-ɣ level in plasma in both group control & autism which indicated significant differences undergo probability value 0.05
Table 7: Correlation between IFN-ɣ Plasma Level and IFN-ɣ Alleles among the Study Group| Group | T allele | A allele | ||
|---|---|---|---|---|
| Frequency | IFN-ɣ con. mean | Frequency | IFN-ɣ con. mean | |
| Autistic group | 22 | 25.75 | 75 | 24.41 |
| Control group | 67 | 17.57 | 85 | 19.30 |
| P. value | 0.01 | 0.01 | ||
ASD are particularly heterogeneous developmental conditions. While extensive work has focused on ASD over the last decade, the implied etiology stills obscure. Many arguments clarified a central function for dysregulation of immune system in ASD and many ASD hazard genes encode the immune system elements along with other risk factors linked to the maternal immune system consisting fatal reactive antibodies, infection, and autoimmunity are correlated with ASD. There is also evidence of continuing dysregulation of immune system in people with ASD and in animal models of this condition. In this study we found that the IFN-ɣ plasma level in autistic patients is highly significant than that in control group, this is in agreement with. Increases in blood cytokine levels from individuals with ASD are also thought to represent increases in the cytokine levels in the brain [20-25].
This finding propose that responses of continues inflammation may be associated to behavioral disturbances and need confirmation in larger frequency studies. IFN-ɣ encourages differentiation of neuron among neural progenitor cells such cells are however untypical and show compromised function and unusual kinds expression of neuronal marker. IFN-ɣ also affects morphology of dendritic and formation of synapse, resulting long term changes in communication and cellular connectivity. Consequently, IFN- ɣ can cause anomalies in synaptic organization by changing the MHCI expression. These studies collectively indicate that exposure to IFN-ɣ directly may trigger unusual neuron developmental, which could explain autism characteristics [25-27].
Regarding the effect of gender on IFN-ɣ gamma plasma level the result showed elevated in IFN-ɣ concentration in both male, female, of autistic patients compared with control group, But the level was higher in the autistic female as compare to the male this may reflect the severity of antisocial behavior in the female than in the male patients. In addition to hereditary influences in ASD etiology, clinical analysis including family and twins studies strongly suggests a substantial role of environmental factors [30- 31].
Previously, analysis of sequence identified several polymorphisms through the IFN-ɣ gene. Such polymorphisms can be found in variant locations within the IFN-ɣ gene, involving (+874 T/A, 764 C/G, 179 G/T) loci. Previously authors published that polymorphism of A/T alleles in the first intron of IFN-ɣ gene supplies a regarding site for binding, which is a factor of transcription ability to promot IFN-ɣ expression. In addition, practical researches indicated the link of Aallel and Tallele with low and high development of IFN-ɣ , respectively [32-34]. However, IFN-ɣ expression can affected by the (+874 T/A) singlenucleotide polymorphism (SNP) [32]. Our result showed that the percentage of A allele was showed excessive in autistic group when compared to control group but this was statistically non significance. On the other hand there is a significance differences in the frequency of T (higher production) allele in the autistic (23.4 %) and control group (67 %), p value < 0.05. However, IFN-ɣ +874 T/A gene polymorphism has been notified to influence its gene expression. Our findings showed that The AA genotype has been associated to decrease IFN-ɣ concentration, contrasted with the TT genotype, while TA genotype was reported moderate percentage.
This suggests that the SNP analysis could be implicated in the ASD developmental. Accordingly, individuals having this allele mutation in gene of IFN-ɣ could have a raised hazard to ASD development. We found that alleles and genotypes repetitive of the IFN-ɣ polymorphism was specific for ASD. The allele was significantly less repetitive in autistic kids than in control children, in this consideration, the relationship between concentration of the IFN-ɣ and genotypes was also notified in a diversity. No reports clarified the correlation between genetic polymorphism of IFN-ɣ and ASD were found.
ASD susceptibility is associated with the T allele of IFN-ɣ (+874 rs2430561 with increase plasma level of IFN-ɣ which may have role in the severity of behavioral changes.
