Author(s): Javed Iqbal*, Abdullah Naeem, Kainat Jahangir, Yumna Ali, Yusra Mashkoor, Ahmer Ashraf, Dalia Mehmood, Maria Mehmood and Lucke-Wold Brandon
Introduction: Hyperbaric oxygen therapy (HBO2) aims to address ischemia resulting from brain injury by subjecting patients to an atmosphere that dramatically raises the concentration of inspired oxygen (100% O2 at greater than 1 ATA). This results in elevated levels of oxygen in the plasma, which in turn boosts the delivery of oxygen for diffusion to the brain tissue.
Objective: To study the efficacy of hyperbaric oxygen (HBO)-based modalities in brain injury
Method: Preferred reporting items for systematic reviews protocol was applied to perform literature search regarding this analytical review.
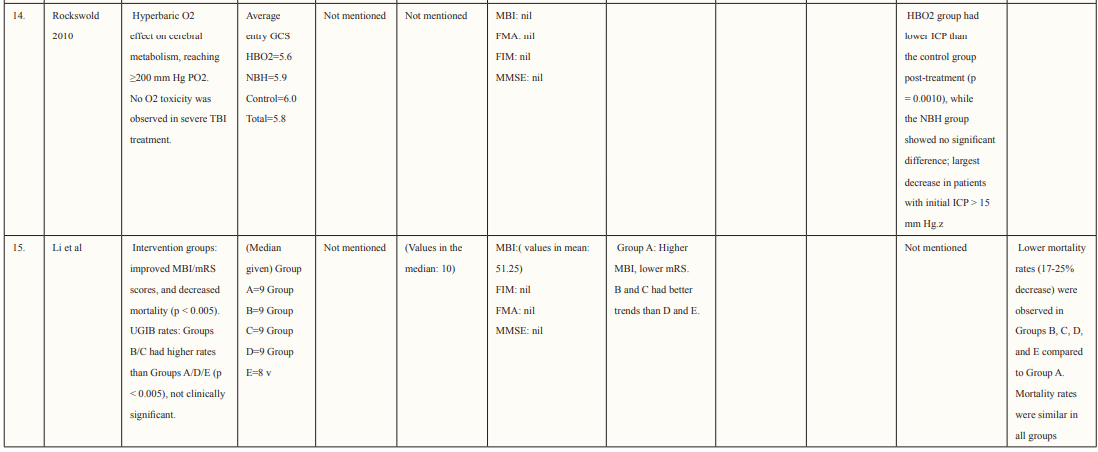
Results: In our study, fifteen studies are included in this review, involving 1067 people. The mean age group of patients enrolled was 57.0±11.6 and the mean NIHSS score was 10.5 ± 8.7, of which 21 participants had moderate to severe neurological impairment. The total number of HBO treatments was 8 to 70 times (28.3 ± 17.9), at the end of the 6-month follow-up period. mRS (modified Rankin scale) ≤3 was found in 25 cases, of which 12 patients with high-grade aSAH recovered. Poor prognosis was prevalent in patients who experienced delayed cerebral ischemia, this was true for 22.7% of patients in this study. In 3 studies conducted by Rockswold, ICP (mm Hg) was significantly lower in the HBO2 group after the treatment than pretreatment. (p<0.05). 4 studies showed an improvement in GCS score after HBO2 therapy.One trial (Imai 2006) reported that three patients in the HBO group died due to pneumonia (two) and heart failure (one) and one patient died in the control group due to heart failure. Overall, it is relatively safe to use HBO in the treatment of brain-related haemorrhage, strokes, and injury as there were no major complications reported.
Conclusion: This systematic review demonstrates that HBO2 has significant clinical potential in treatment of brain related haemorrhages, stroke and injury.
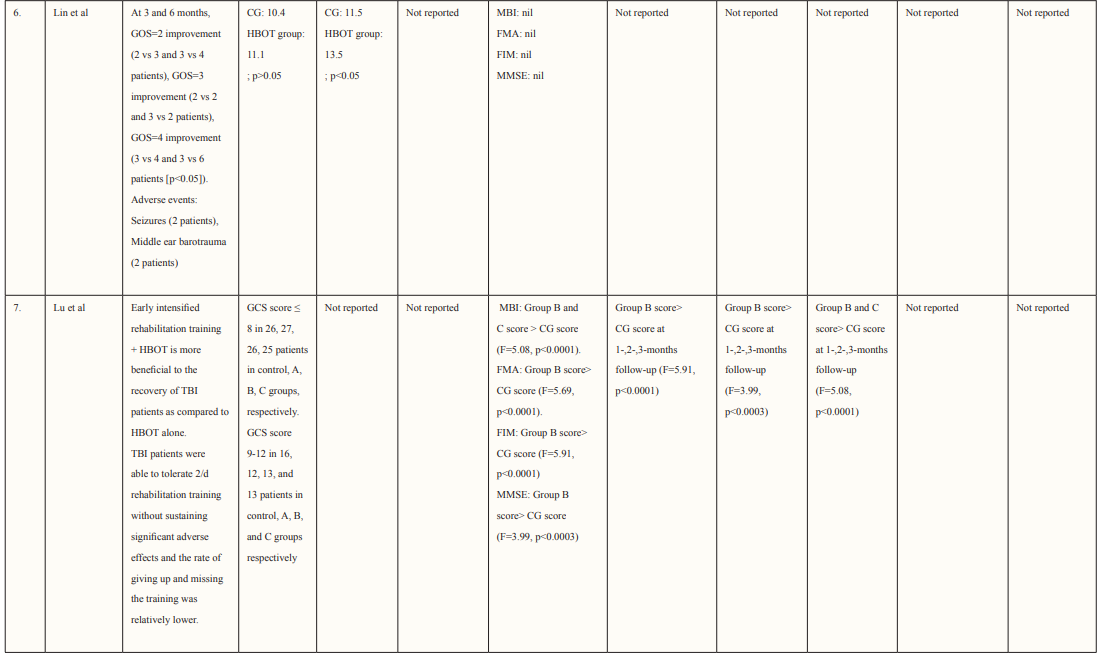
Stroke is rapidly becoming one of the leading causes of mortality worldwide, with only coronary heart disease ranking higher. Moreover, every year, the number of individuals being diagnosed with stroke is increasing drastically, and what is even more concerning is the fact that people are being diagnosed at a much younger age than ever before. The medical community recognizes three main categories of stroke, which include ischemic, intracerebral hemorrhage, and subarachnoid hemorrhage. Despite various treatment options available to stroke patients, managing the condition remains challenging due to its high prevalence,expensive treatment costs, and the fact that many patients have a poor prognosis. [1-3]
The main objective of treating acute ischemic stroke (AIS) is to restore blood flow to the brain tissue, which is essential for the survival of brain cells [4]. Although early administration of intravenous thrombolysis (IVT) and endovascular therapy (EVT) are effective in achieving revascularization, they do not always guarantee a favorable outcome for the patient. Despite these interventions, the prognosis for stroke patients remains challenging and uncertain [5-7].
Hyperbaric oxygen therapy (HBO) is a treatment method that involves inhaling 100% oxygen while inside a pressurized hyperbaric chamber that is set to a pressure level greater than 1 atmosphere. This therapy is commonly used in the treatment of various neurological disorders such as global and focal cerebral ischemia, vegetative states, cerebral vasospasm after subarachnoid hemorrhage, and carbon monoxide poisoning. Studies have shown that animals treated with HBO after ischemia have experienced improved neurological outcomes and reduced brain edema [8-11].
The therapeutic effects of HBOT are attributed to several mechanisms, including increased oxygen delivery to tissues, improved tissue oxygenation, neovascularization promotion, wound healing enhancement, inflammation reduction, and neuroprotection [12-17].
Experimental studies indicate that HBOT mitigates early brain injury after SAH, possibly by inhibiting hypoxia-inducible factor- 1α and its target genes. HBOT has also been found to suppress oxidative stress by reducing NADPH oxidase enzymatic activity, lipid peroxidation, and neuronal damage. Furthermore, HBOT has been utilized to lower intracranial pressure (ICP) in SAH cases and related CVS. However, caution is advised when interpreting results due to the potential rebound effects of HBOT, which may pose risks, especially for patients with high ICP or those on artificial respiration [18].
A comprehensive and quantitative review of the literature on hyperbaric oxygen therapy (HBOT) and brain injury outcomes is crucial to inform clinical decision-making, guiding future research, and identifying knowledge gaps. SAH, a severe form of hemorrhagic stroke, is associated with high morbidity and mortality rates [19]. Research has shown potential protective effects of HBOT in both preclinical studies and in various SAH cases as well. [20-22].
This systematic review has been reported in concordance with the guidelines provided by Preferred Items for Systematic Review and Meta-Analysis (PRISMA) [23]. The PRISMA checklist is designed and presented in the Supplementary file.
The literature search was conducted systematically on the Pubmed/ Medline, Google Scholar, and Cochrane library databases, with the MeSH terms: “hyperbaric oxygen” OR “hyperbaric oxygen therapy” OR “HBOT” OR “oxygen therapy” AND “traumatic brain injury” OR “TBI” OR “hemorrhage” OR “subarachnoid hemorrhage” OR “SAH” OR “intracranial hemorrhage” OR “intracerebral hemorrhage” OR “stroke” OR “hematoma”, used in various combinations by applying a timeframe from 2000 to 2023. After database search and removal of duplicates, the shortlisted studies were screened by two reviewers, using title and abstract; only studies in the English language were selected, and those performed on the human specimen. Exclusion criteria were implemented on the remaining articles, discarding studies after full-text screening based on irrelevance, poor quality, and ineligible outcomes. In addition, the cited references of studies were manually searched for any qualifying articles. During the screening process, if the reviewers found the eligibility of an article controversial and a mutual agreement could not be reached, a third independent reviewer would be approached to settle the confusion.
The criteria for inclusion and exclusion of studies were fixed after discussion with the authors. Only randomized controlled trials (RCTs) and observational studies were included in the analysis that involved clinical or pre-clinical outcomes of hyperbaric oxygen therapy in adult TBI patients including those hospitalized for subarachnoid hemorrhage, any intracranial bleeding, and/ or cerebrovascular event. Poor-quality trials, letters to editors, commentaries, case reports, cross-sectional studies, conference posters, proceedings, and personal communications were excluded. The further exclusion was based on overlapping study populations, text in languages other than English, and non-availability of sufficient data. Clinical studies that involved patients with post- concussion syndrome or post-concussive symptoms and non- traumatic brain injuries such as ischemic, anoxic, or cryogenic damage, were excluded from the review.
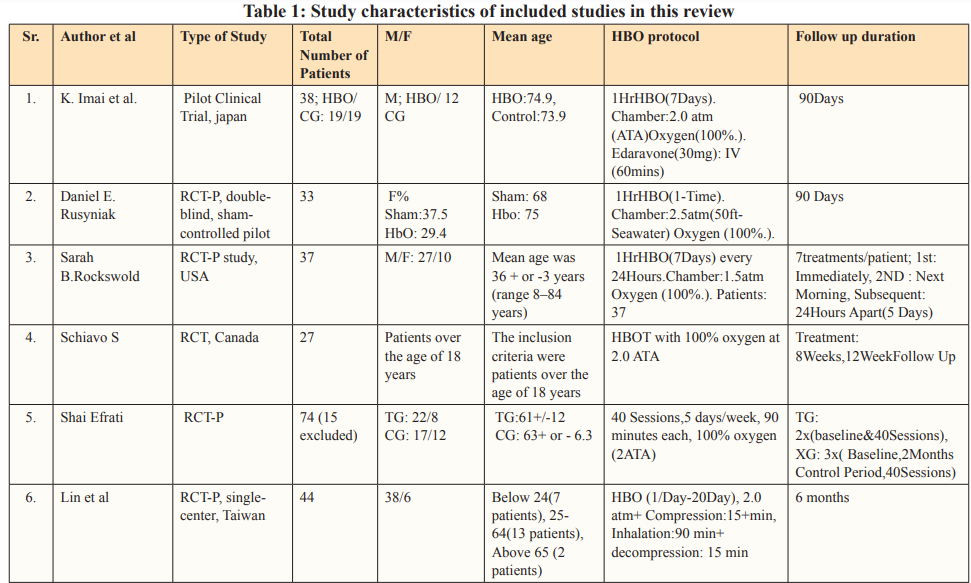
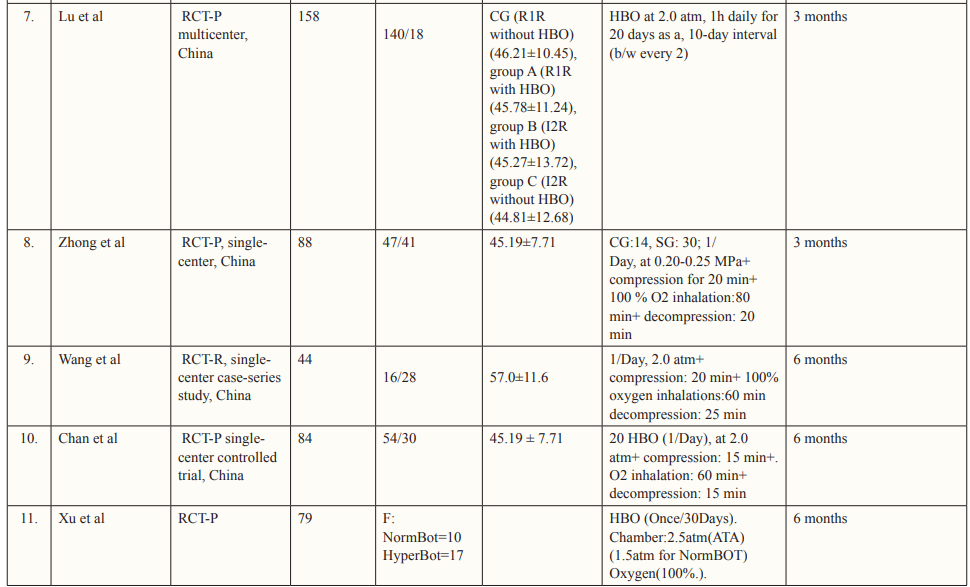
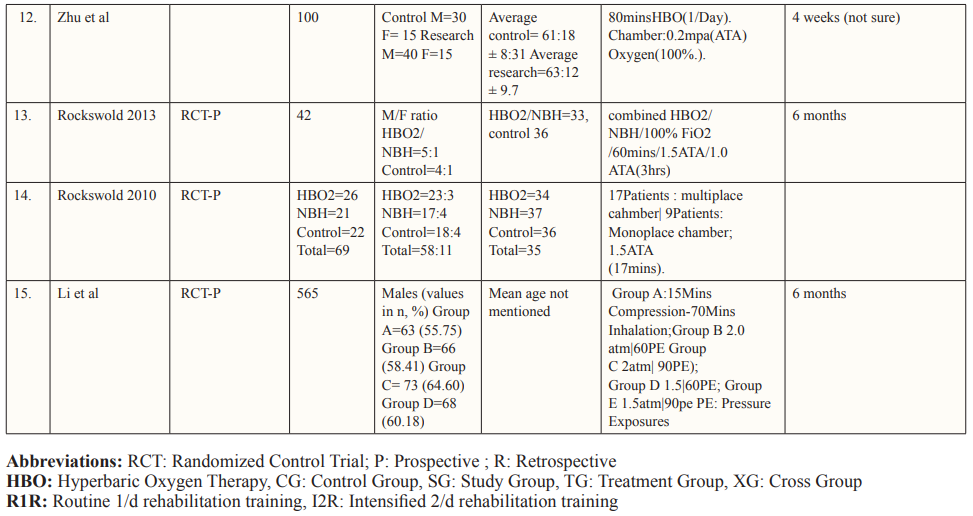
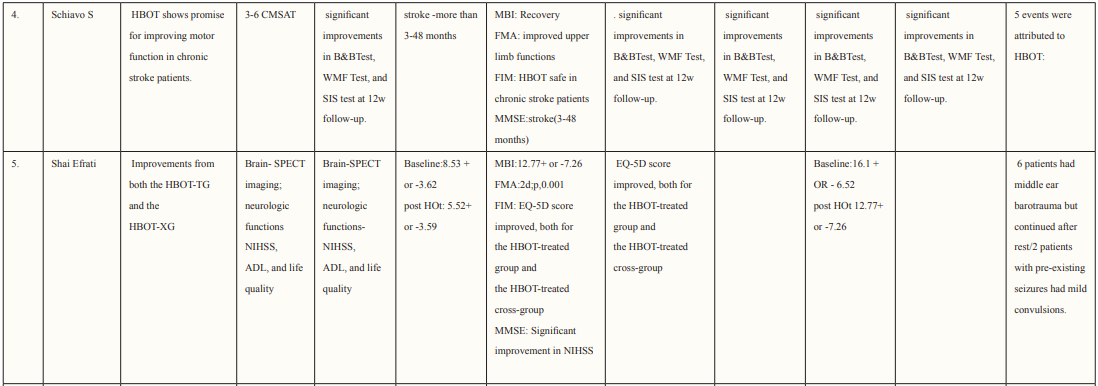
The authors performed data extraction on characteristics of each study and its patient population including study design, country of study, the total number of patients, age and gender of patients, mechanism of injury, hyperbaric oxygen treatment protocol, pre- and post-HOBT Glasgow Coma Scale (GCS) scores, follow-up duration, and the reported outcomes from each study that met the inclusion criteria. The study characteristics of each study are described in Table 1.
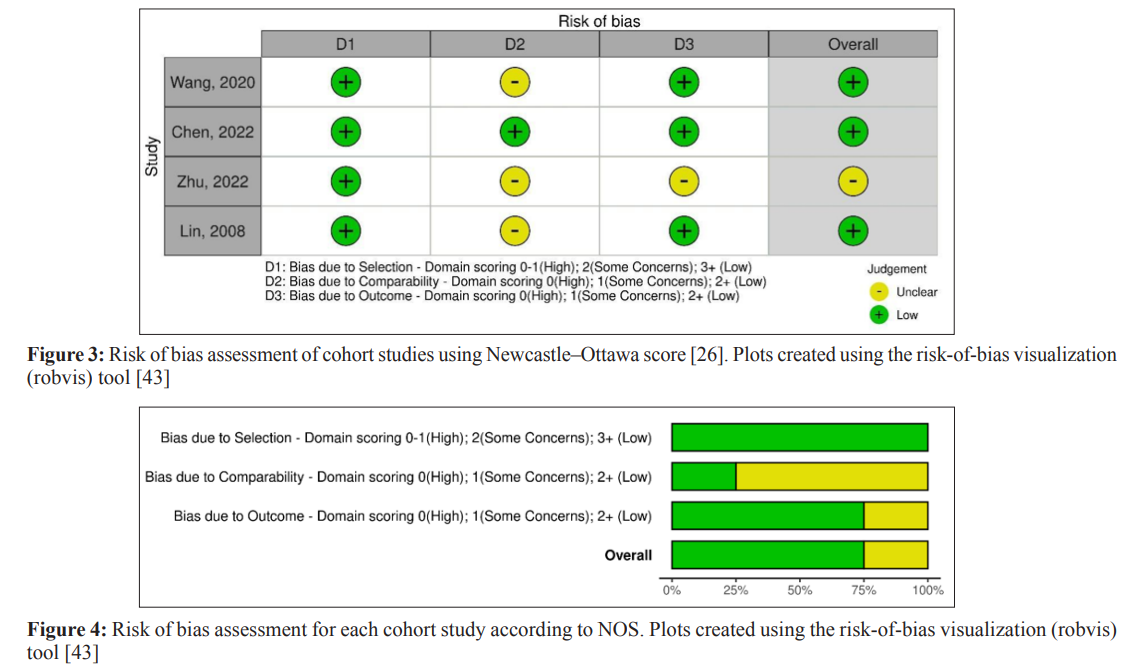
The quality of each study was interpreted by measuring the risk of bias for RCTs using the Cochrane Collaboration Risk of Bias tool [24]. The performance bias, selection bias, reporting bias, detection bias, attrition bias, and other biases were estimated for each RCT. We scored the risk of bias in each category as low, high, or unclear, as shown in Figure 2. In a large number of the included RCTs, the probability of performance bias was high as blinding of the surgeons, investigators, and patients was not executed. Some trials showed a low risk of selection bias whereas all the RCTs showed a low risk of attrition and reporting bias. As far as the observational cohort studies are concerned, the risk of bias was assessed using the Newcastle Ottawa Scale, in which the cumulative scores in selection, comparability, and outcome sections in the scale identify the quality of each observational study as good, fair, or poor [25]. Most of the studies were scored as good quality, as summarized in Figure 3.
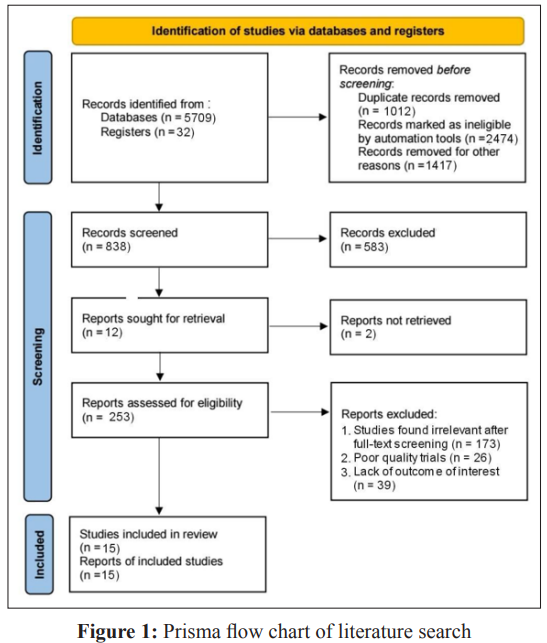
The PRISMA flow chart summarizes the search and study selection process (Figure 1). In our initial search, we identified 5741 studies from PubMed using the keywords hyperbaric oxygen, hyperbaric oxygen therapy, HBOT, oxygen therapy, traumatic brain injury, TBI, hemorrhage, subarachnoid hemorrhage, SAH, intracranial hemorrhage, intracerebral hemorrhage, stroke, and hematoma. After removing duplicates, 838 studies remained, and we excluded 583 studies based on title and abstracts. We accessed the full text of the remaining 253 studies, we excluded 173 based on the exclusion criteria (selection criteria table), 26 as they reported poor-quality trials, and 39 for lack of outcome interest. Ultimately, we included 15 studies. Of all the included articles, 11 were randomized control trials [26-36] and 4 were cohort studies [37-40]; all 15 articles were from Japan, USA, Canada, Israel, Taiwan, and China. Study characteristics and baseline characteristics of participants are provided in Tables 1 and 2.
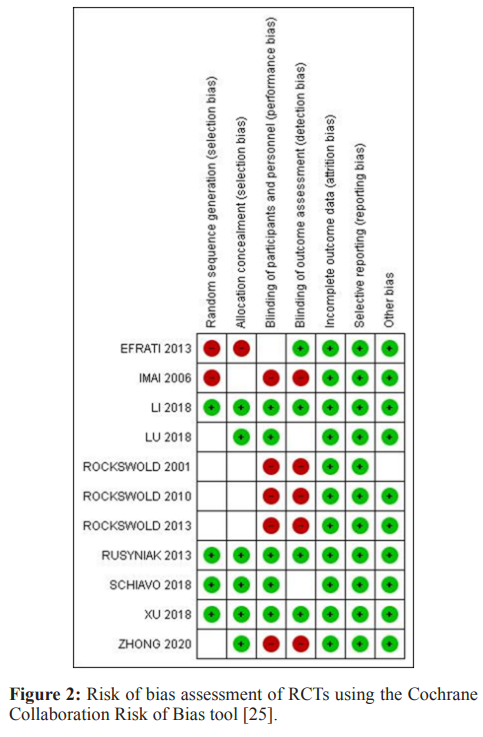
We assessed the risk of bias for each clinical trial using the Cochrane Collaboration Risk of Bias tool [24] (Figure 2).
Fifteen articles had a total of 1,482 participants who were eligible for data pooling according to the data extraction. Most of the included studies reported multiple aspects of their trials to appraise the risk of bias assessment which was a predominantly low risk of bias. 5 trials reported adequate detail to indicate a low risk of bias [26, 28, 35, 39, 41].
Four trials randomly assigned patients either to the intervention or control group, these were deemed to be trials with a low risk of bias [25, 27, 33, 34]. The remaining trials did not report how they randomized or allocated their patients into their respective groups hence we labeled these trials as unclear risk of bias.
Four trials randomly assigned patients either to the intervention or control group, these were deemed to be trials with a low risk of bias [25, 27, 33, 34]. The remaining trials did not report how they randomized or allocated their patients into their respective groups hence we labeled these trials as unclear risk of bias.
Allocation concealment (selection bias) was reported in 6 trials, these trials reported the allocation of concealment and hence were classified as low risk of bias [26, 28, 32, 34, 34, 39]. On the other hand, 4 trials did not mention did not provide any information on this domain so were classified as having unclear risk of bias [29-31, 33]. Lastly, one trial did not adhere to the allocation of concealment and hence was classified as a high risk of bias [36].
Blinding of participants and personnel was reported in 5 trials and therefore we classified them as low risk of bias [26, 28, 34,35, 39]. 5 trials reported that participants were not blinded so we classified them as high risk of bias [29-33]. One trial did not mention whether participants were blinded to the treatment group and hence was classified as an uncertain risk of bias [36].
In four trials outcome assessors were blind to participant group allocation therefore we classified them as low risk of bias [26, 28, 34, 36]. 5 trials reported that outcome assessors were not blinded to participant group allocation hence we classified them as high risk of bias [29-33]. Lastly, two trials did not provide any statement about blinding of outcome assessment and so were classified as having unclear risk of bias [35, 39].
All included trials reported that there were no withdrawal of participants or loss to follow-up that appeared in the analysis in any of the trials hence all trials were considered low risk of bias for this domain.
We classified all trials as low risk of bias because protocols were available for each trial.
Using the Newcastle-Ottawa Scale (NOS), two separate writers evaluated the methodological quality and bias risk of the cohort studies that were included [25]. The NOS evaluates the effectiveness of non-randomized research’ designs, including cohort and case-control studies. An overall score out of nine was given for the selection criteria, comparability, and result (cohort) or exposure (case-control). According to the NOS score, the risk of bias in the overall study was rated as high, moderate concern, or low (Figure. 3,4). If any of the three domains (selection criteria, comparability, or result) obtained a high risk of bias grade, the study was assessed to have a high risk of bias overall.
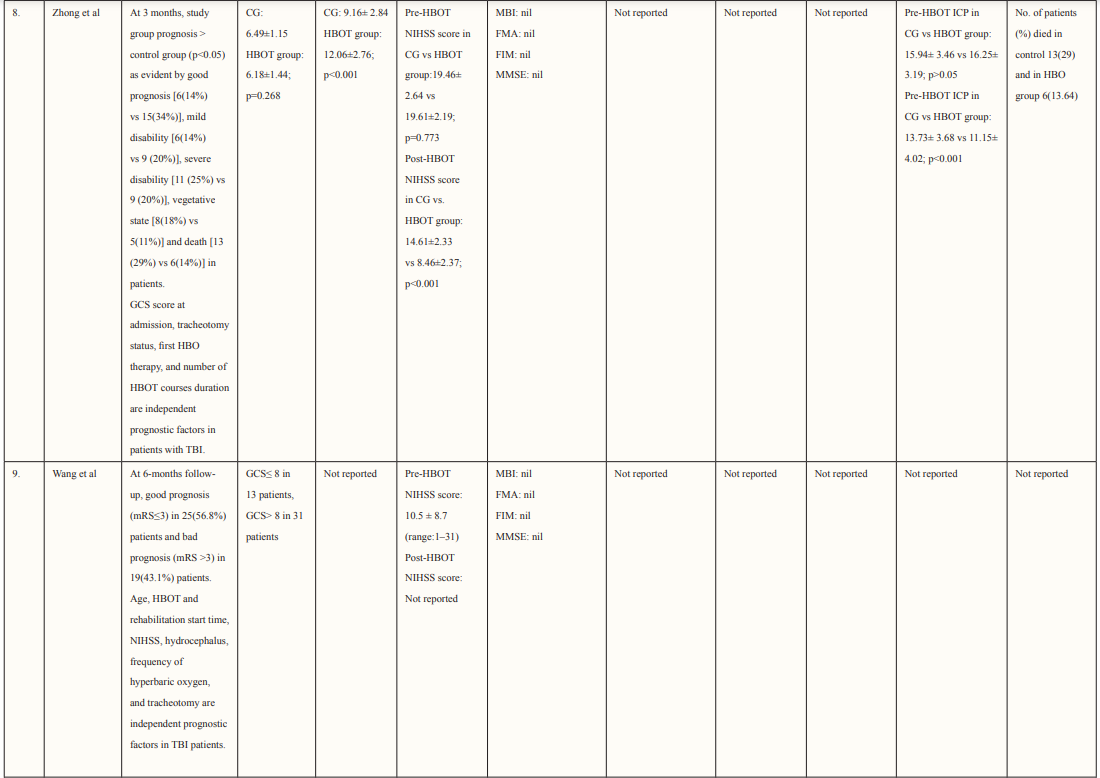
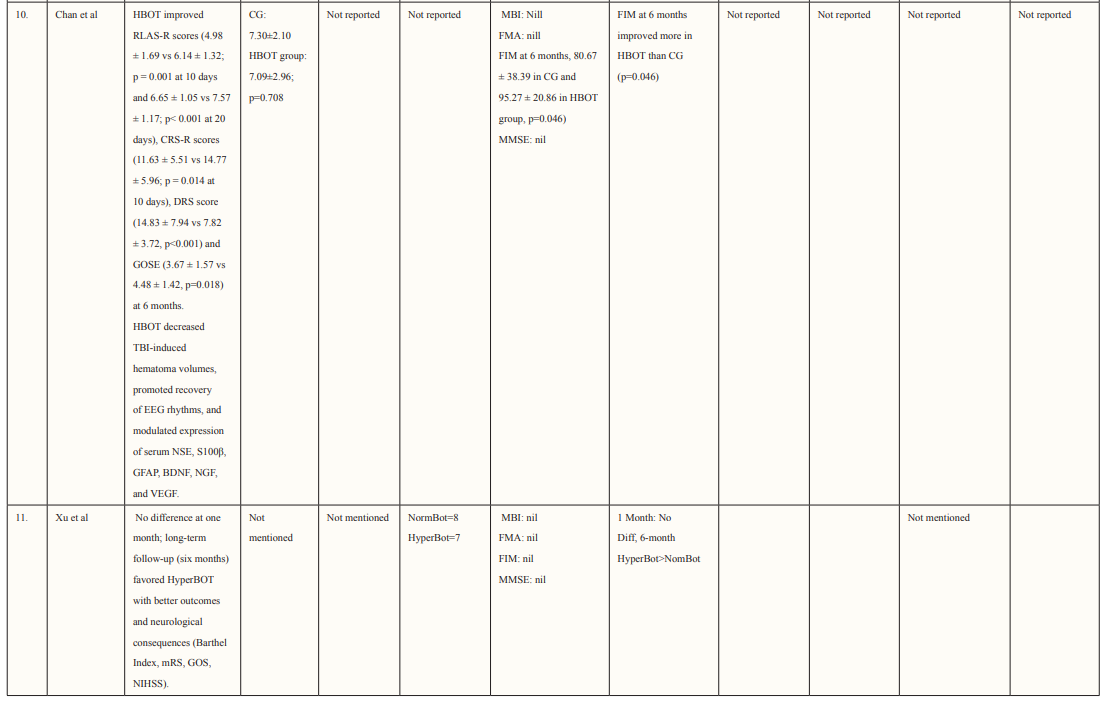
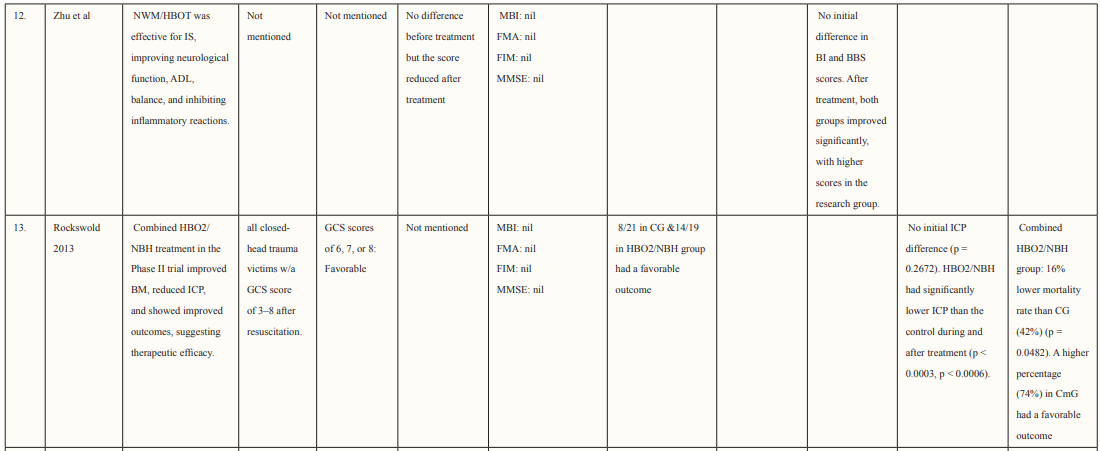
Most of the included studies were of good quality and had a low risk of bias. Studies were conducted in various countries including Japan, USA, Canada, Israel, Taiwan, and China. The outcomes assessed for each study are described in Table 2. Six studies supported the use of HOBT in traumatic brain injuries (TBI); 4 studies concluded the effective use of HOBT for ischemic strokes; one supported the use of HOBT in hemorrhagic stroke; one approved the use of HOBT in intracerebral hemorrhage; one reported the effectiveness of HOBT in patients with acute subarachnoid hemorrhage (aSAH) and one displayed the effectiveness of HOBT with Edaravone in patients with acute embolic stroke. The studies showed that most patients’ conditions improved after receiving several sessions of HOBT hence can be deemed to be an effective measure in the management of patients who have had a subarachnoid hemorrhage to improve health outcomes and reduced mortality. The results in most studies showed that patients were given 100% oxygen, this was safe and favorable for patients who suffered from either TBI, ischemic, embolic, or hemorrhagic stroke, intracerebral, and acute subarachnoid hemorrhage. The complication related to treatment were minor, many of the studies reported that patients experienced transient otalgia, hypertension and tachycardia, lower back pain, claustrophobia, minor seizures treated with anticonvulsants, and middle-ear barotrauma. Although, one trial reported that three patients in the HBO group died due to pneumonia (two) and heart failure (one) and one patient died in the control group due to heart failure [33]. Overall, it is relatively safe to use HBO in the treatment of brain-related hemorrhage, strokes, and injury as there were no major complications reported. Many of the studies did not have a large sample size hence it is difficult to conclude the true adverse effect of HBO therapy, adverse effects should be studied further in a larger sample size. Wang et al. determined the use of HBO therapy on patients with subarachnoid hemorrhage treated with rehabilitation treatment. The mean age group of patients enrolled was 57.0 ±11.6 (16 males and 28 females), and the mean NIHSS score was 10.5 ±8.7, of which 21 participants had moderate to severe neurological impairment. The total number of HBO treatments was 8 to 70 times (28.3 ±17.9), at the end of the 6-month follow-up period. mRS (modified Rankin scale) score was used to determine the long-term functional prognosis, mRS ≤3 (good prognosis) points were found in 25 cases, of which 12 patients with high-grade aSAH recovered. Poor prognosis was prevalent in patients who experienced delayed cerebral ischemia, this was true for 22.7% of patients in this study.
The use of HBOT in the treatment of hemorrhage is long been evaluated for its effectiveness and efficacy. Subarachnoid hemorrhages account for 5-10 % of all strokes and hemorrhage is more common in the young population. The mortality rate in SAH is quite high and around 1/3rd of the patients have long-lasting disabilities after the hemorrhage [19]. Historically, Hyperbaric Oxygen (HBO) therapy was not commonly administered during the early stages of Subarachnoid Hemorrhage (SAH) and was only considered useful in cases where patients experienced cerebral vasospasm after the bleeding in the subarachnoid space.
In this study, we focused on the efficacy of hyperbaric oxygen therapy in treating subarachnoid hemorrhage. This systematic review included a total of 15 studies including 11 randomized control trials and 4 cohort studies. In the systematic review, we found that HBOT is an effective treatment option for patients with different types of brain injuries including traumatic brain injury, intracerebral hemorrhage, embolic stroke, ischemic stroke, and subarachnoid hemorrhage. In addition, most of the included studies have shown that HBOT could significantly improve patient conditions after several sessions of HBOT. The studies that are included in the review are conducted in multiple countries and are at low risk of biased.
In all the included studies no withdrawal of the participants or loss to follow-up periods was reported. While certain weakness of the trial includes the complications that were reported by patients including otalgia, hypertension and tachycardia, lower back pain, claustrophobia, minor seizures treated with anticonvulsants, and middle-ear barotrauma. While more severe complications including some life-threatening ones were reported in the studies. In the study, there have been 3 deaths that were reported in the HBOT group two due to pneumonia whereas one of the deaths occurred after heart failure. Whereas one patient in the control group also died of heart failure [33]. Whereas in the study of one death of the patients occurred before the follow-up period [34]. in one of the studies included in the review by the patient outcome was not determined as the study was not related to treating the patient and addressing the outcome. 2 of the studies reported mild to moderate barotrauma in the ear and chest pain [30, 35, 36].
No direct comparison can be made between the present systematic review and any previous review as we found none. There is a possibility of publication bias as only the published data were included. The studies included in the review did not have long- term follow up so long-term outcomes of the therapy could not be assessed. Although this study is the first to explore the effect of hyperbaric oxygen therapy in patients with subarachnoid hemorrhage, there are certain limitations to this systematic review which weaken the strength of retrieved evidence mainly due to the small sample size and different populations involved. Although the review points towards that the HOBT is an effective treatment option in patients with subarachnoid hemorrhage, the overall quality of evidence for the effective treatment of subarachnoid hemorrhage by HOBT therapy is still low due to the small study population in the review. The study protocols also vary from study to study with different follow-up duration and the treatment that is provided which further limits the efficacy of the review.
