Author(s): Sunil Palchaudhuri
Pneumocococcus or S.pneumoniae, a member of Gram-positive Streptococcus Mitis group is capable of causing several severe diseases like bacterial pneumonia, meningitis, bronchitis, otitis media (mid-ear infection). In order to stop these diseases, the preventive therapy appears to be an appropriate option because of our antibiotic resistance crisis born in 1960 in Tokyo Hospital (Japan) in the treatment of Shigellosis has not yet been solved. The causative pathogen Shigella flexneri is a close kin of our non-pathogenic E.coli K-12. In the absence of any alternative therapy, our clinicians have been increasing the doses of antibiotics with longer duration but now in 2022 we are almost standing against the wall. How about our vaccines? That could have been a real solution but the antigenic variation of this bacterial pathogen may not guarantee the successful treatment. Did we try to understand how such variants of the pathogen arise? One possibility appears to be a collaborative effort of bacteria and their bacteriophages (viruses). We have extensively studied lysogeny with E.coli K-12 and its virus bacteriophage lambda. Such a lysogenic state may not be limited to just bacterial pathogen but the virus COVID-19 (RNA genome) in our respiratory cells may be responsible for their antigenic variation.
Introduction
In 1928, Dr Fred Griffith observed two kinds of bacterial colonies
on blood agar medium media during incubation for 48 hours
after the collection of blood samples from his patients with lobar
pneumonia [1]. His laboratory note book recorded some details
of his follow up. However, this pathogen S.pneumoniae grows
very slowly even on blood agar media. During such incubation
the small or Smooth colonies grew large but with uneven contour
(his note book describes it as Rough colony).
We lost him in World War II and 1944 Avery et al made an attempt to understand the difference between these colonies (Smooth and Rough) as recorded in his note book but went after DNA (TCA insoluble precipitate) without the knowledge of double helix DNA and bio-macromolecule [2,3]. Apparently these investigators picked up colonies of S.pneumonie and then the lysates were sheared to reduce the viscosity , used alkaline pH to get rid of RNA’ and then changed to neutral pH. The TCA precipitate was the DNA genetic material but these are nucleotides and far away from the genes and genomes. In fact our knowledge about bio-macromolecules grew gradually but the difference between individual bacterium and its colonies was also important to appreciate Dr Griffith’s two types of bacterial colonies on his blood agar medium. Unfortunately, until 1953 our double helix DNA was not recognized as DNA bio-macromolecule [3,4]. Now is the time to understand its growth curve and make an attempt to stop such a serious pathogen by an alternative therapy in the presence of antibiotic resistance crisis. In microbiology text books this pathogenic S.pneumoniae (bacterium) has been defined as diplococcic but not in the competent phase. Although the diplococcic shape is real only in their late stage or overnight culture. We should also recognize that this pathogen starts as a small round shape (coccus), then looks oval and then the oval to diplococcic but not any permanent structure. I want to make it clear that the same bacterium changes only its morphological look during its growth in pre-competent, competent and post- competent phase. Even our mother looks very different in her late stage of pregnancy but this bacterial pathogen allows their progeny to prevail in their chain until they are competent to communicate via bio-signalling in their community. This article describes the progeny of S.pneumoniae growing in their pre-competent phase after release from the spheroplast by the shearing force induced.
In my work previously published I have made an attempt to describe how this progeny are evolved. In this context I In 1964 three other investigators at NYU School of Medicine have purified this Gram-positive pathogen as S.pneumoniae and grew them in a liquid medium almost to stationary phase (post-competent phase or spheroplasts. Then fine structure analysis. Precisely these S.pneumoniae population, mostly spheroplasts were sliced with a diamond knife for direct visualization under electron microscope at a very high magnification (almost 170,000X) [4]. This article is the knowledge I needed to go for its real growth curve in later years (1990 - 2014). Meanwhile the subject grew as molecular Microbiology; Molecular Genetics confirms the difference between DNA nucleotide (Avery et al.) and bio-macromolecule (genes and genome) was highly important regardless of its presence in Gram positive Streptococcus or Gram negative E.coli K-12 [5,6].
Result and Discussion
DNA is our genetic material, not only in prokaryotes but also
in eukaryotes. There are two kinds of genetic transformation in bacterial genetics-Natural transformation (growth curve in three
phases, pre-competent, competent and the post-competent) and
the artificial transformation (mostly used in in vitro gene cloning
experiments using multi-copy antibiotic resistance plasmids as
cloning vectors! The artificial transformation has been mostly
used in in-vitro gene cloning experiments with Gram-negative
E.coli K-12 and the cloning vectors used for what ! . What are
these cloning vectors? Answer is straight forward that these are
mini-R-plasmids carrying antibiotic resistance transposons. Our
gene cloners were not aware about its dreadful consequences.
What is worse, Dr Stanley Cohen was the medical faculty of
Stanford University (the PI) and Annie Chang is his laboratory
technician. Dr Cohen, and a learned boss but even in his recent
article published in PNAS. I now want to go back and try to
define NATURAL TRANSFORMATION.
Natural transformation has not yet been recognized in an appropriate manner and therefore the growth curve of Streptococcus pneumoniae is still hovering in darkness. It seems to me that the natural transformation is really the growth curve of S.pneumoniae that grows in three phases - pre-competent phase. competent and the post-competent. There is heterogeneity of sizes and shapes even in their pre-competent phase indicating all their members are not born simultaneously. For our ignorance we have not taken precaution required to save the spheroplast population, individually carrying their progeny.
Figure 1: shows the pre-competent population in heterogeneity after the rupture of the spheroplasts of the Mitis group Streptococci. After dilution of overnight culture in the research laboratory by pipetting (or Eppendorf) the progeny is released from spheroplasts.
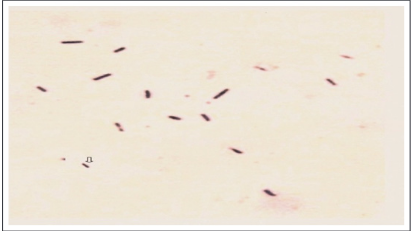
Figure 1. Mitis group Streptococcus progeny released when overnight culture grown without shaking and then diluted by using glass pipettes. Progeny sizes and shapes confirm their growth in heterogeneity. Gram-staining technique modified knowing the heterogeneity of such a population in precompetent phase, competent phase and post-competent phase (spheroplast).
The majority of these bacterial population end up in post- competent phase and morphologically appear diplococcic. These are the mothers of Streptococcus progeny but pregnant with their live progeny and we therefore should avoid the shearing force. Unfortunately the growth curve is still not well defined but is that genetic material DNA/RNA does not differ regardless of virus or elephant. Let me walk backward to appreciate that we learn from our mistakes. In 1964 there was an important article published showing the genesis of such progeny but they were biased by the Avery et al (1944) and the abuse of the word DNA started without the knowledge of DNA as bio-macromolecule of Watson and Crick (1953). However, these authors were miserably biased and unfortunately they closed their eyes when they almost visualized the whole truth but by sectioning the population with diamond knife but growth curve was not available then. However, they were misled by accepting the word DNA as if DNA means the bio-macromolecule. ignorance helped these mothers who are really carrying the progeny the members are adult but does not mean that the entire population are mothers). The heterogeneity also prevails in this phase obviously we should not think that all the population even being in competent phase may not carry the progeny). Therefore, growth curve is required to stabilize the entire population of the pathogen even they prevail in their inherent heterogeneity. We are now presenting the growth curve of Streptococcus pneumonia but accepting the truth that the pre- competent phase is mostly the progeny released by the lysis of spheroplasts. The Spheroplast represents the post-competent phase and morphologically, is really the mother with the live progeny and therefore looks diplococcic. Usually we diluted our overnight cultures of S pneumoniae(spheroplasts) in fresh medium by pipetting or Eppendorf and the population of spheroplasts are lysed by such shearing force releasing the progeny in the growth medium. The progeny thus released consist of individual members in heterogeneity of shapes and sizes. This is the population in pre-competent phase. Fig.1 shows this population in heterogeneity.In 1972 our article published in the proceedings National Academy Sciences USA, after the recommendation of Nobel prize winner Professor Severo Ochoa. We have made it very clear that the TCA insoluble precipitate of DNA as reported by Avery et al in 1944 is really collapsed nucleotides of DNA fragments. These double stranded DNA fragments were formed at random after their alkaline denaturation of chromosomal DNA of S.pneumoniae and renatured by neutralizing the pH. This was also true in the isolation of Gram-negative E.coli K-12 chromosomal DNA (Palchaudhuri Sunil,unpublished data) . Therefore, I had to modify the existing DNA-isolation procedures to visualize the chromosome or extra-chromosome (F-plasmid in super-coiled covalently closed circular DNA) [7,8].
Because of our modification of existing Gram -staining technique to visualize the entire population in the pre-competent phase the morphological look of spheroplasts in diplococcic shape (mother carrying progeny) was sacrificed to some extent. The crystal violet solution was not completely washed with acetone -alcohol mixture. However, the progeny in heterogeneity of growth become barely visible but looked pink to pinkish. The spheroplasts, fully lysed by the complete rupture looked as pink- bush adjacent to another spheroplast not lysed but they still prevail in chain (not shown).
Acknowledgement
I acknowledge the availability of Dr Anubha Palchaudhuri, MD,
Ph.D. for frequent discussion and computer expertise of Tripti
Bhattacharya in the preparation of this article.
References
1. Griffith, F (1928) The significance of pneumococcal types.
J. Hyg. 27: 113-159.
2. Avery, O.T,MacLeod CM and McCarty M(1944). Studies on
the chemical nature of the substance inducing transformation
of Pneumococcal types. J.Exp.Med. 79: 137.
3. The pneumococcus, ASM book 2004.
4. Snyder L and Champness W (2007) Molecular Genetics of
Bacteria (3rd ed).ASM Press, Washington, D.C.
5. Palchaudhuri S, Palchaudhuri A, Chatterjee B. (2016) Growth
curve of Streptococcus oralis. EJBR. 6: 36-41.
6. Watson JD, FHC Crick (1953) Molecular structure of nucleic
acids. Nature 171: 737-738.
7. Palchaudhuri S, Mazaitis AJ, Mass WK, Kleinshmidt AK
(1972) Characterization by electron microscopy of fused
F-prime factors in Escherichia coli. Proc.Natl.Acad.Sci.USA.
69: 1873-1876.
8. Tomasz A, Jamieson J.D, Ottolenghi E (1964) The fine
structure of diplococcus pneumoniae. The journal of cell
biology 22: 453-467.
