Author(s): MD Lugos*, A Dangana, BD Ntuhun, BO Oluwatayo and OD Damulak
Follicular lymphoma (FL), a non-Hodgkin lymphoma, is an indolent cancer of the B cell lineage that runs a chronic deterioration course that can result in multiple treatment episodes leading to resistance and possible transformation to diffuse large B cell lymphoma. Cytomegalovirus (CMV) reactivation during chemotherapy or after an organ or hematopoietic stem cell transplantation is a major cause of morbidity and mortality. This study tests the hypothesis that some of the heterogeneity of FL might result from chronic infection with Cytomegalovirus (CMV). This research was intended to appraise the impact of CMV infection on the subtypes of T cells in follicular lymphoma patients. We accessed stored peripheral blood mononuclear cells (PMBCs) from patients of known CMV serostatus recruited into an FL clinical trial. We undertook a multicolour flow cytometric analysis of the PBMCs and compared the number of lymphocyte subtypes of CMV-positive and CMV-negative FL patients. Data showed a significant increase in the quantity of terminally differentiated (TEMRA) T cell subsets, including EM3-CD8 (P=0.005), EM3-CD4 (P=0.018), E-CD4 (P=0.029), E-CD8 (P=0.033) and pE2-CD4 (P=0.046) phenotypes, as well as increased NKT cells (P=0.031) among CMV-positive patients compared to the negative group. Our findings support the hypothesis that recurrent infections characterise CMV infection in FL due to accelerated immune senescence and the accumulation of exhausted T cells. Based on the data, a case could be argued for the routine application of CMV screening in FL before treatment with chemo-immunotherapy to implement enhanced infection surveillance in CMV-positive patients. These discoveries can eventually help improve the treatment approaches in the management of FL toward a combinatorial viewpoint for direct cytotoxic and indirect immunomodulatory outlook.
Introduction
A variety of cell types and subtypes such as granulocytes,
macrophages, dendritic cells, T cells, and B cells at different
populations constitute an essential part of the immune system [1].
In CMV infection, a large fraction of T cell subtypes have been
reported to be committed to keeping the virus under checks, thereby
causing alterations in the dynamics of T-cell populations, which
strongly influence T cell immunity [2]. Therefore, quantitation,
defining and ascribing specific roles played by the diverse T cell
populations, for instance, in the immunity of different pathological
conditions, will help provide diagnostic and prognostic evidence
for patient management in FL Multicolour flow cytometry can be
used to quantify different subtypes of T cells and other cell types.
Further research evidence has explained the events leading to
cellular differentiation, acquisition of effector potential and
eventual death of T cells. Activation of immature CD4+ T cells
has been reported to present a linear differentiation model, in
which cells continuously acquire functional abilities by every
additional differentiation step [3]. However, persistent antigenic
provocation over time can result in a gradual decline of memory
and cytokine secretion capacities, and consequently, accumulation
of short-lived CD4+ T cells (TEMRA cells) with poor cytokine
production ability. Furthermore, other antigenic assault on the
CD4+ TEMRA cells can activate the CD4+ T cell at this stage
of differentiation, causing apoptosis. On the other hand, two
alternative differentiation patterns have been reported to produce
CD8+ TCM cells and CD8+ TEM cells: linear differentiation and
fixed lineage [4, 5]. A continued antigenic challenge will lead to
accumulation of CD8+ TEMRA cells and death similar to the CD4+ T cells pattern; however, CD8+ TEM cells can regain
IL-2 expression and become CD8+ TCM cells, unlike CD4+
TEM cells [4]. NK cells are a subcategory of lymphocytes of the
innate immune system that form about 10% of the circulating
blood mononuclear cells in humans they are usually described as
CD3-CD56+ cells and can be subgrouped by the intensity of CD56
on the cell surface [6,7]. The distinct subtypes of NK cells can
be classified based on CD56 and CD16 surface markers [8]. The
majority of circulating NK cells (about 90%) exhibit low expression
for CD56 (CD56dim) with high levels of CD16 and perforin and
are described as terminally differentiated NK cells [9]. NK cell
development is characterised by the reduction in the expression of
CD56 and the addition of CD16 expression. However, activated
CD56dimCD16+ has been shown to represent the highest level
of NK cell activation, which implies that CD56dimCD16+ cells
represent the most mature NK cells subtype [10-12]. Therefore,
this study was set out to evaluate the impact of CMV infection on
circulating peripheral T and NK cell populations in FL patients.
Materials and Methods
The flow cytometer - BD LSRFortessa™
Cells were acquired and analysed using the BD LSRFortessa™
Special Order Research Product (BD Biosciences, Oxford, UK).
It is an air-cooled multi-laser benchtop flow cytometer, equipped
with Red, Yellow/Green, Blue, Violet and UV lasers, capable of
analysing 18 parameters. The equipment is housed in the Liverpool
Good Clinical Laboratory Practice (GCLP) Facility, Liverpool.
The Multicolour FACS Design
We used a 3-tube multicolour FACS analysis design to quantify
several T and NK cell subtypes in the peripheral mononuclear
blood cells (PBMCs) of FL patients, as illustrated in Figure 1.
Tube 1 contains the ten fluorochrome-conjugated monoclonal
antibodies (mAbs) listed in Figure 1, designed to analyse for
senescent T cells (CD57+CD28-), NKT and NK cells (CD56 &
CD16), B cells (CD19+) and monocytes (CD14+) of the study
cohort. Tube 2 contained 12 mAbs and was dedicated to examining
the frequencies of the different differentiation stages to T cells,
including the naïve (CD45RA+CD45RO-CCR7+CD62L+),
central memory (CD45RA-CD45RO+CCR7+CD62L+),
effector memory (CD45RA-CD45RO+CCR7-CD62L-) and
effector (CD45RA+CD45RO-CCR7-CD62L-) T cells. Also, we
evaluated terminally differentiated T cells (TEMRA) subtypes
using CD27 and CD28 as well as those of regulatory T cells (Tregs)
(CD4+CD25+). Tube 3, an 11 mAbs panel, committed to analysing
T cell exhaustion markers, including PD-1, LAG-3, and TIM-3.
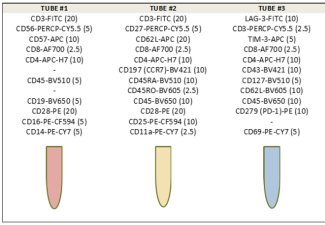
Figure 1: Illustrating the 3-tube FACS design showing the
respective panels of Fluorochrome-Conjugated Monoclonal
Antibodies (mabs).
The values in brackets represent the respective optimal working
volumes of the mAbs (in microliters) as validated in our lab.
The Fluorochrome-conjugated Monoclonal Antibodies (mAbs)
A total of 26 fluorochrome-labelled monoclonal antibodies
were used for this study, including CD3-FITC (BD Biosciences,
Oxford, UK), CD3-PerCP-Cy5.5 (BD Biosciences, Oxford, UK),
CD4-APC-Cy7 (BD Biosciences, Oxford, UK), CD8-Alexa
Fluor 700 (BD Biosciences, Oxford, UK), CD11a-PE-Cy7 (BD
Biosciences, Oxford, UK), CD14-PE-Cy7 (BD Biosciences,
Oxford, UK), CD16-PE-CF594 (BD Biosciences, Oxford,
UK), CD19-BV650 (BD Biosciences, Oxford, UK), CD25-PE-
CF594 (BD Biosciences, Oxford, UK), CD27-PerCP- Cy5-5
(BD Biosciences, Oxford, UK), CD28-PE (BD Biosciences,
Oxford, UK), CD43-BV421 (BD Biosciences, Oxford, UK),
CD45-BV510 (BD Biosciences, Oxford, UK), CD45-BV650 (BD
Biosciences, Oxford, UK), CD45RA-BV510 (BD Biosciences,
Oxford, UK), CD45RO-BV605 (BD Biosciences, Oxford, UK),
CD56-PerCp.-Cy5.5 (BD Biosciences, Oxford, UK), CD57-APC
(BD Biosciences, Oxford, UK), CD62L-APC (BD Biosciences,
Oxford, UK), CD62L-BV605 (BD Biosciences, Oxford, UK),
CD69 -PE-Cy7 (BD Biosciences, Oxford, UK), CD127-BV510
(BD Biosciences, Oxford, UK), CD197 (CCR7)-BV421 (BD
Biosciences, Oxford, UK), CD279 (PD-1)-PE (BD Biosciences,
Oxford, UK), LAG3-FITC (Enzo Life Sciences, UK), and TIM3-
APC (R & D Systems, UK). We also had CD45- BV650 & BV510
isotype (BD Biosciences, Oxford, UK) controls. Fluorochrome-
conjugated mAbs were kept in the fridge until usage.
Sample Size Estimation
Sample size calculations were carried out based on pilot data
collected before the start of the main study. The previous
investigation found that 8 out of the 27 cytokines to be studied
were shown to show a significant difference between CMV-
positive and CMV-negative patients with FL. Based on replicating
these results, sample size calculations are based on a Bonferroni
adjusted alpha level of 0.00625 to control the overall type I error
rate at 0.05. As cytokines are measured on different levels, sample
size calculations assume the data are measured on the standardised
normal distribution. It was determined that difference would be
interesting if the difference in cytokine expression levels between
positive and negative CMV were at least one standard deviation.
Given that each cytokine is measured on a different scale, each cytokine will have its standard deviation. Therefore, the variables used for the sample size calculation for comparison between two groups with an equal number of patients include the statistical power at 80%, an alpha level at 0.00625, a standard deviation of 1 and a clinically relevant difference of 1. This gives an overall sample size of 42 patients.
Study Population and Samples
This study benefited from 42 stored pretreatment PBMC samples
from FL patients recruited into an FL phase 3 clinical trials,
which compared two alternative frontline chemoimmunotherapy
regimens comprising 21-CMV- positive and 21-CMV negative.
The study samples were stored at -80oC before use. Aside from
the strict inclusion and exclusion criteria applied in the recruitment
of FL trial participants, a careful selection of study patients based
on balanced clinical and pathological baseline characteristics as
in Table 1 between the CMV groups was adopted. This approach
aimed to minimise the possible influence of confounders in the
analysis and enhance the chances of accurate evaluation of the
biological effect of CMV infection status on FL.The demographic and pretreatment features of the study population are described in Table 1. CMV- positive patients are marginally
older compared to CMV- negative with a median age of 73 years versus 69 years (p=0.055). Patients’ risk factors and the prognosis
are stratified according to the Follicular Lymphoma International Prognostic Index (FLIPI) score into three risk categories: good
(0 or 1), Intermediate (2) and poor (3-4). The FLIPI score is determined using five adverse prognostic factors, including Ann Arbor
stage (III-IV vs I-II), age (> 60 years vs ≤ 60 years), haemoglobin level (< 120 g/L vs ≥ 120 g/L), number of nodal areas (> 4 vs ≤
4), and serum LDH level (above normal vs normal or below) [13]. A slightly higher proportion of CMV- positive patients is on the
high-risk grade compared to the CMV- negative (71% vs 67%). The Cumulative Illness Rating Scale (CIRS) is used for the physical
assessment of impairment of patients under 13 near independent areas. It is a 5-point rating of the degree of severity scale that ranges
from none (1) to extremely severe (5) [14]. The proportion of patients in Table 1 did not show a significant difference due to CIRS.
Also, as shown in Table 1, there is no significant difference in the clinicopathological variables of patients included in the two arms
of study cohorts, the CMV- positive and CMV- negative.
Table 1: The comparison of pretreatment features by CMV infection status of patients whose PBMC samples were analysed
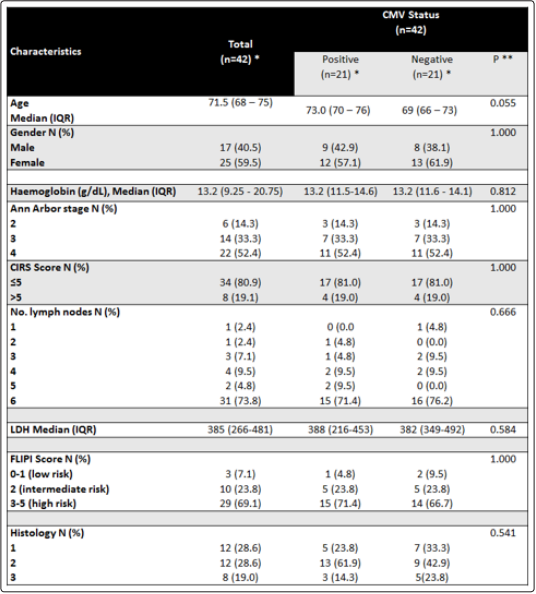
For continuous variables, this refers to the Mean (SD) or Median (IQR) if indicated **P-value by x2 test or Fisher’s exact test for the difference between categorical variables or t-test for the difference between two means.
Inclusion and Exclusion Criteria
The inclusion criteria for recruiting patients into the National Cancer Research Institute (NCRI) PACIFICO (Purine-Alkylator
Combination In Follicular lymphoma Immuno-Chemotherapy for Older patients) phase III clinical trial includes established grades 1,
2, and 3a patients of mainly 60 years or above, at Ann Arbor stages II to IV without prior treatment, as well as minimal haematological
and other health complications. The exclusion criteria can be summarised to include Grade 3b FL and over, cases of transformed FL
to DLBCL and other health complications that may not withstand the adverse effects of chemotherapy.
Ethical Approval & Informed Consent
As part of the PACIFICO trial ethics application, this study was
included in the context of translational research. This trial had
approvals of the European Union Drug Regulating Authorities
Clinical Trials (EudraCT) on a unique number 2008-004759-
31 and the International Standard Randomised Controlled Trial
(ISRCTN) number ISRCTN99217456. Written informed consent
to participate in the NCRI PACIFICO trial was obtained before
recruitment to the study as per the International Council for
Harmonisation (ICH)-Good Clinical Practice (GCP) regulations.
The laboratory techniques
The Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
In a biological class II Safety Cabinet, Lymphoprep (Product #:
1114740, Axis-Shield, Alere Ltd., Stockport-UK) was pipetted
into prelabeled tubes in a 2:1 ratio of blood to Lymphoprep. Blood
was gently layered on top of the Lymphoprep using a serological
pipette. The tubes were centrifuged at 800g for 30 minutes at
room temperature (RT) with the brake setting at low. Following
centrifugation, mononuclear cells in the buffy coat layer were
carefully removed into a tube, which was filled up with Roswell
Park Memorial Institute (RPMI)-1640 medium (LM-R1641/500,
Labtech International Ltd, East Sussex, UK) and centrifuged at
550g for 10 minutes to wash and pellet the cells with the brake on.
The supernatants were discarded, and cells were resuspended in the
appropriate volume of freezing solution A (10% foetal calf serum
(FCS) in RPMI 1640 media. Cells were counted to determine
live and dead cells using ChemoMetec Nuclear Counter in the
GCP Lab facility, and an equal volume of medium B (10% FCS,
20% Dimethyl sulfoxide (DMSO in RPMI) was added slowly.
Cells were aliquoted in vials and placed in Nalgene cryofreezing
containers in the -80oC freezer for a minimum of 12 hours before
moving to -150oC freezer in the GCP Lab Facility freezer room.
The Cytometer Setup and Tracking (CS&T)
The CS&T is a fully automated BD FACSDiva software and
reagent research system unique to BD digital cytometers designed
to provide Characterisation, Setup, and Tracking for baseline
settings. This system optimises and standardises cytometer setup
and tracks cytometer performance, ensuring consistency and
reproducibility of FACS data by offsetting the routine instrument
variability. The BD FACSDiva CS&T Research Bead set (Cat #:
655051, BD Biosciences, Oxford UK) consists of uniform beads
of different intensities (bright, mid, and dim beads) designed
to characterise the flow cytometer fully. For daily performance
checks, a CS&T passed result was considered essential for running
the FACS experiment for the day.
The Compensation Set up
After vortexing the BD™ CompBeads(Cat #: 552843, BD
Biosciences, Oxford UK) had one drop each of the negative
control and anti-Mouse Ig, κ was added to each of 12 prelabelled
compensation tubes representing each fluorochrome. The optimal
working volume of each primary conjugated antibody was added
to the respective tube. The tubes were gently mixed and incubated
for 30 minutes in the dark at RT. Subsequently, 2ml of CellWash
(Cat# 349524; BD Biosciences, Oxford UK) was added to each
tube and centrifuged for 5 minutes at 300 × g with a low brake
setting to wash beads. Supernatants were discarded, and the bead
pellets were resuspended in 500μL BD FACSFlowTM Sheath
Fluid and vortexed thoroughly before analysis. Five thousand
events were acquired from each of the 12 compensation tubes.
By using the software, the bead population was carefully gated,
and the positive and negative populations were identified before
the compensation values were calculated using BD FACSDiva
software version 7.
The PBMC Samples: Thawing and Recovery of Cells When ready to use, the frozen specimens were thawed by being held in a closed fist before transferring the cells to pre-label Universals on ice. Each 1mL of thawed cell suspension was slowly diluted with 10mL of RPMI- 1640 medium supplemented with 10% foetal calf serum (FCS). The RPMI 1640 solution was added to cells gradually in a drop-wise manner with constant and careful agitation throughout the process. The Universals were centrifuged at 500 × g for 5 minutes, and the supernatants were discarded.
Then the cell pellets were resuspended, counted and assessed for viability using ChemoMetec NucleoCounter (ChemoMetec A/S, Denmark) by detecting total and dead cell counts. Cells were resuspended in BD FACSFlow Sheath Fluid (Cat #: 342003, BD Biosciences, Oxford UK) for lymphocyte immunophenotyping.
The Staining and Acquisition of Cells for Facs
Following the addition of optimal working volumes of the
respective fluorochrome-conjugated monoclonal antibodies (data
not published), 100μL containing about 2 × 105 to 1 × 106 well-
mixed washed PBMCs were placed in the prelabelled FACS
tubes. Tubes were incubated for 30 minutes in the dark, at room
temperature, after which 2ml of CellWASH (Cat #:349524, BD
Bioscience Oxford, UK) was added, and the tubes centrifuged
at 300g for 5 minutes with low brake. The supernatants were
discarded before repeating the washing process. Finally, the cells
were resuspended in 500μL of BD FACSFlowTM Sheath Fluid
before being gently vortexed.
Due to variations in the availability of material and the number of gated T cells (CD3+), the number of events acquired per tube was between 100,000 and 2,000,000, with most samples yielding 500,000 events and above. Isotype controls and single stained fluorescence histograms were used to gate for cut-offs for distinct lymphocyte populations on the Diva software platform using respective surface markers.
The Hierarchical Illustrations of the Approach used for the
Analysis of Cell Types
A PACIFICO trial stored PBMC sample, pac0108, was used
to illustrate the contour plot gating approach and the hierarchy
adopted to analyse the subsets of lymphocytes. We used FACSDiva
software, which provides ready information on the number of events
(#Events), per cent parent cell (%Parent), and per cent total (%Total)
as displayed on illustrations of hierarchical analysis of different
cell types in tubes 1-3 depicted in figures 2-4 respectively. In all
three tubes, gating began by identifying the CD45+ population.
For instance, in tube 1, four main populations were gated from the
common leukocyte antigen (CD45-Amcyan), including CD3-Alexa
Fluor-488 (T cells), CD14-PE-Cy7 (Monocytes), CD19-Qdot-655
(B cells) and CD56-PerCP (NK cells).
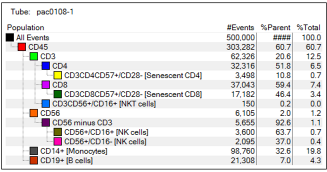
Figure 2: The hierarchical approach adopted to analyse cell types tested in tube 1 shows the #Events, %Parent and %Total
pac108 -1 represents a PACIFICO trial stored PBMC sample used to illustrate the hierarchy adopted to analyse cell types in tube 1. The cell types of interest are represented in brackets.

Figure 3: The hierarchical approach adopted for analysis of cell types tested in tube 2, showing the #Events, %Parent and %Total
pac108 -2 represents a PACIFICO trial stored PBMC sample used to illustrate the hierarchy adopted for the analysis of cell types in tube 2. The cell types of interest are represented in brackets.

Figure 4: The hierarchical approach used to analyse cell types tested in tube 3 shows the #Events, %Parent and %Total
pac108 -3 represents a PACIFICO trial stored PBMC sample used to illustrate the hierarchy adopted for the analysis of cell types in tube 3.
The number of events, per cent parent and per cent total events of the various cell types and subtypes were defined automatically and displayed using FACSDiva software. Conventionally, this provides the needed values for measuring the per cent of lymphocyte subpopulations within the lymphocyte gate. A comparative analysis of the median values of per cent parent (relative) frequencies of the different lymphocyte subtypes according to the CMV status was carried out using a nonparametric Mann-Whitney U-test analysis to determine the significant difference. A p-value of < 0.05 is considered significant.
Results
The analysis of cell surface markers has been used to define the
various lymphocyte populations in stored PBMC samples using
multiparametric flow cytometry. Essential FACS optimisation
steps aimed at minimising errors and generating reliable results
and subsequent interpretations were strictly adopted. Of note,
the right approach to FACS data analyses and presentation of
the cell population of interest is as crucial as cells acquisition
design. Before the FACS experiments commence, optimal working
volumes of fluorochrome-conjugated antibodies were determined,
contour and dot plots were evaluated against polygon and rectangle
gating strategies, respectively, gating strategies for the three FACS
tubes were evaluated, and fresh versus frozen FACS assessed.
The Lymphocyte Subtypes
Using the established flow cytometry method and analysis
strategies, we successfully quantified relative percentages of
T and NK cells from parent cells (as in Figures 2,3 and 4) in
samples taken from a cohort of patients with FL (see Table 2).
Of interest, statistically significant elevation of effector (E) and
effector memory (EM) T cell subtypes, representing end-stage
CD4 and CD8 T cell populations among CMV-positive patients
compared to the CMV-negative counterparts was observed.
Statistically significant higher frequencies of pre-effector-2 CD4
(pE2-CD4; CD45RA+CD27+CCR7-CD28-), effector-CD4 (E-
CD4; CD45RA+CD27-CCR7-CD28-), effector memory-3 CD4
( EM3-CD4; CD45RO+CD27-CCR7-CD28-), effector-CD8 (E-
CD8; CD45RA+CD27-CCR7-CD28-) and effector memory-3-
CD8 (EM3-CD8; CD45RO+CD27-CCR7-CD28-) phenotypes
were observed in CMV- positive compared to CMV-negative
patients as shown in Table 2. These T cell subcategories’ most striking common features are the loss of CD28 and CCR7 (CD28-CCR7-)
surface markers. The subtypes of effector cells express CD45RA+ isoform; those of the effector memory cells express CD45RO+.
Also, looking at the results in Table 2, a statistically significant increase in the frequency of NKT cells (p=0.031) was detected among CMV-positive patients compared to CMV- negative cohort. However, NKT cells are known to perform both protective and destructive consequences due to their abilities to produce cytokines that support either inflammatory responses or immune tolerance.
The other cell types with marginal differences between CMV groups include CD4+ T cells population and CD4-LAG3 (p=0.051 and p=0.074; Table 2). Also, a marginal difference is observed in the ratio of CD4+ and CD8+ T cell populations between CMV- Positive and CMV- negative groups (1.2 vs 1.5, p=0.054; Table 2).

The table shows median relative percentages of cells types as determined from the parent populations in the hierarchical procedure in Figures 2, 3 and 4. The cell markers analysed included CD4, CD8, the ratio of CD4 to CD8 (CD4: CD8) calculated, senescent CD4 (Snt-CD4), CD8 (Snt-CD8), natural killer T cells (NKT cells), monocytes, B cells, Naïve CD4 (N-CD4), pre-effector - 1 CD4 (pE1-CD4), pre-effector - 2 CD4 (pE2-CD4), effector CD4 (E-CD4), central memory CD4 (CM-CD4), effector memory -1 - CD4 (EM1-CD4), effector memory -2 - CD4 (EM2-CD4), effector memory -3 - CD4 (EM3-CD4), effector memory -4 - CD4 (EM4-CD4), regulatory T cells (Tregs), naïve CD8 (N-CD8), pre-effector - 1 CD8 (pE1-CD8), pre-effector - 2 CD8 (pE2-CD8), effector CD8 (E-CD8), central memory CD8 (CM-CD8), effector memory -1 - CD8 (EM1-CD8), effector memory -2 - CD8 (EM2-CD8), effector memory -3 - CD8 (EM3-CD8), effector memory -4 - CD8 (EM4-CD8), Programmed cell death protein- 1 CD4 (PD1-CD4), PD1-CD8, Lymphocyte-activation gene -3 CD4 (LAG3-CD4), Lymphocyte-activation gene -3 CD8 (LAG3-CD8), T-cell immunoglobulin and mucin-domain containing-3 CD4 (TIM3-CD4), T-cell immunoglobulin and mucin-domain containing-3 CD8 (TIM3-CD8), natural killer cells (CD56+/CD16+ and CD56+/CD16-).
The CD4+ T cells have shown a reduced frequency in the CMV- positive FL patients than CMV- negative group. Notably, CD4+ T cells alongside CD8+T cells forms the bulk of T-lymphocytes. The CD4+T cells are critical in attaining a coordinated, efficient immune response to pathogenic agents. Following activation, CD4+T cells differentiate into distinct effector subtypes that actively mediate immune response by secreting different cytokines and performing many functions. The functional activities of CD4+ T cells cover the activation of the cells of the natural immune system, B-lymphocytes, cytotoxic T cells, and nonimmune cells, and also play a crucial part in the elimination of immune response. Although no striking observation is made on the values of exhaustion surface markers analysed, higher expression values of CD4+ and CD8+ PD-1, LAG-3 and TIM-3 (Table 3-3) phenotypes are seen among CMV- positive cohort compared to CMV- negative arm. The CMV-negative group presents a marginal increase in the median value of CD4: CD8 ratio of 1.5 against 1.1 of CMV- positive FL patients.
Also, no significant difference in quantities is observed of senescent CD4+ and CD8+ T cells in the study population. However, the CMV-positive patients show an increased expression trend for senescent CD4+ and senescent CD8+ T cell populations compared to the negative FL patients.

Figure 5: Boxplots of end-stage T cell subtypes and NKT cells showing significant difference between CMV-positive and -negative patients
Discussion
The FACS analysis of the stored PBMC samples of FL patients has revealed high quantities of subtypes of end-stage terminally
differentiated (TEMRA) T cells from both the CD4+ and CD8+ T cell compartments as well as NKT cells in the CMV-positive patients
compared to the CMV- negative group. In details, these data show that pre-effector-2 (pE2) (CD45RA+CD27+CCR7-CD28-), effector
(CD45RA+CD27-CCR7-CD28-) and effector memory-3 (EM3) (CD45RO+CD27-CCR7-CD28-) subtypes of the CD4+ T cell
compartment, effector (CD45RA+CD27-CCR7-CD28-) and effector memory-3 (EM3) (CD45RO+CD27-CCR7-CD28-) phenotypes
of the CD8+ T cell compartment accumulated more in the CMV-positive patients. Phenotypically, the terminally nonproliferating
effector T cell subsets usually lack the costimulatory receptors CD27 and CD28 as well as lymph node homing receptors CCR7 and
CD62L on the cells surface, and are variously referred to as TEMRA, CD45RA+ memory, terminally differentiated (TTD) or end-
stage or persisting effector T cells [15]. As a result, these cells are characterised by diminished long-term memory potential, limited
effector function and poor cytokine production, resulting in impaired immune functions, leading to increased vulnerability to infections.
CD4+ and CD8+T cells constitute most T-lymphocytes in the
peripheral blood, both of which possess specific T cell receptors
for efficient adaptive immunological responses. The high
accumulation of nonproliferating versions of these cells portends
immune deficits for the patients. Moreover, effector T cells that
display TEMRA phenotypes have been reported to demonstrate
senescence and end-stage differentiation and are found eminently
increased in both CD4+ and CD8+ T cell arms, especially in
association with ageing and CMV infection [16, 17]. A close
look at the differentiation phases of T cells identifies terminal
effector T cells (TEMRA) as the most differentiated subtype of
memory T cells, which are known to be extremely susceptible
to apoptosis and display high levels of perforin and Fas ligand
(cytotoxic molecules) [18].
Although a high proportion of NKT cells is observed in the CMV- positive FL patients, a functional analysis of these cells would be required to help in assessing the functional potential of the high-frequency cells in FL However, NKT cells are known to share properties of both NK cells and T cells. They act rapidly to stimulus, a characteristic of the innate arm of the immunity, and can produce cytokines such as IL-2, IFN-gamma, TNF-alpha, and IL-4 typical of adaptive immunity to promote or suppress different immune responses [19]. Of interest, the NKT cells have been shown to play a role in tumour immunity. Notably, it has been demonstrated that poor IFN-gamma production due to defective NKT cells was observed in patients with progressive malignant multiple myeloma but not in non-progressive myeloma or premalignant gammopathy [20, 21]. Furthermore, an analysis of peripheral blood has reported a statistically significant reduction in the quantities of NKT cells in patients with some solid tumours in relation to healthy persons [20, 22]. Also, decreased production of IFN-gamma or proliferation by the type-I NKT cells associated with cancer patients and low circulating levels of type I NKT cells was an independent predictor of poor overall survival (O/S) and disease-free survival (DFS) in patients with head and neck squamous cell carcinoma [23].
Conversely, the type II NKT cells mediate an opposite regulatory role in tumour immunity; for instance, activation of type II NKT cells by sulfatide enhanced tumour burden. However, because we did not stratify the NKT cells into their subtypes, it is impossible to conclude which subtype of NKT cells are elevated in the CMV-positive FL patients and the impact of such an increase in these clinical outcomes patients. However, drawing from the functional profile of NKT cells, it is also sensible to infer that the CMV-positive FL will either suffer more recurrent infections and adverse events (AEs) or poor response to therapies. It is also suggested that the increase in NKT cells in CMV- positive cases might be a compensatory response to the reduction in adaptive immunity resulting from reduced CD4+ T cells and accumulation of exhausted T cells.
Contrarily, although marginally significant, elevated CD4+T cells (p=0.051, Table 3-3) were associated with CMV-negative FL patients compared to the CMV-positive group. Of note, CD4+ T helper (TH) and cytotoxic CD8+ T lymphocytes (CTL) constitute the bulk of T-lymphocytes. Importantly, CD4+ T cells that comprise different lineages elicit immune responses via the production of distinct cytokines and perform multiple functions, including activating a host of immune and nonimmune cells such as B-lymphocytes and cytotoxic CD8+ T cells. In this regard, the CMV- negative FL patients possibly have some immunologic advantage over the CMV-positive patients. Furthermore, although there is no significant difference in the quantities of the Naïve- CD4+ and -CD8+ T cell phenotypes due to CMV status, the CMV- negative patient have shown a trend of higher median values for both naïve CD4+ and CD8+ T cells compared to the CMV- positive group (Table 2). Finally, it is essential to mention that having sufficient quantities of naïve T cells provide a more virile response and protection against infections by novel pathogens [24].
The multicolour flow cytometric analysis employed by the current study seems to demonstrate for the first time an analysis of a large number of subpopulations of T cells in FL, especially in relation to CMV infection. However, to secure a clear-cut interpretation of flow cytometric analysis of circulating lymphocyte subtypes, a functional analysis of the various T cell subsets is suggested. In the meantime, an exploratory analysis relating the quantities of the cell types to clinical data, involving treatment response status and toxicities records of the trial patients will help provide a further understanding of the clinical implications of findings. Also, it is a well-known fact that in a deliberate effort to keep the CMV under checks, the immune profile of a latent CMV-infected individual is adversely affected, particularly leading to an expanded population of CMV-specific memory CD8+ T cells, in a memory inflation phenomenon [25]. Unfortunately, it was not within the scope of this study to investigate this important hallmark of CMV infection by evaluating for CMV-specific CD8+ T cells and functional studies that would have further support the findings.
Although not much has been reported on CD4 TEMRA cells, the phenomenon of expanded end-stage T cells in the circulating peripheral blood has been reported by researchers in relation to other clinical conditions. For instance, a study by Reinke et al. has reported a significant correlation between elevated levels of end-stage CD8 (+) effector memory T (TEMRA) cells in peripheral blood with delayed fracture healing [26]. This study has provided a clear illustration of the role of T cells in modulating endogenous bone fracture repair even in the absence of infection. Another study that employed the Cox regression model reported a 2-fold higher risk of late graft dysfunction among kidney transplant recipients who had increased levels of differentiated TEMRA CD8+ T cells before transplantation [27]. Also, an increased accumulation of terminally differentiated CD8 T cells (TEMRA) has been reported in chronic graft-versus-host disease (cGVHD) as a consequence of continuous differentiation from naïve/central memory T cells to TEMRA cells following prolonged alloantigen exposure [28]. However, a study that also determined the frequency of subtypes of peripheral T-cells, including CD8 TEMRA cells before kidney transplantation, showed that advanced end-stage renal disease (ESRD) related with T-cell dysregulation is associated with a relative expansion of CD8 TEMRA cells guards against the acute rejection of kidney allografts [29]. Elevated percentages and absolute numbers of TEMRA cells in the subsets of CD4 and CD8 T that express CD26 were observed in type 1 diabetes, and the number of TEMRA cells associated positively with indices of intermediate and long-term glycaemic control [30]. The significant accumulation of TEMRA T cells in type 1 diabetes patients is thought to be caused by a long-term and sustained antigenic stimulation consequent to a prolonged presentation of viral or other antigenic particles or could be a homeostatic deficit in the regulation/contraction of immunological responses.
Judging from the significant accumulation of end-stage T cells and high senescence and exhausted T cells in the CMV-positive FL patients, one may speculate that the presence of CMV infection in FL patients can alter the immune dynamics of patients. Thus, these findings support the hypothesis that CMV infection results in perturbation of the immune system in FL in a way that might influence disease course. These revelations can be factored in the management approach for Follicular lymphoma patients towards an improved outcome
Acknowledgements
We thank Dr Melanie Oates (PhD), Dr Ke Lin (Bachelor of
Medicine, MD, PhD) and Professor Andy R Pettitt (MA, MB
BChir, PhD, MRCP, FRCPath), who supervised and provided
reagents for this study. We are thankful to Dr Richard Jackson
& Miss Silvia Cicconi of Biostatistics Department, Institute of
Translational Medicine, University of Liverpool, Mr Philip Jalo
of the Intercountry Centre for Oral Health (ICOH) Africa Jos,
Nigeria, for helping out with statistical analysis. The content of
this manuscript has been published [IN PART] as part of the thesis
of Moses Dabah Lugos [31].
References
1. Lu P, et al. (2015) Supervised learning methods in modeling
of CD4+ T cell heterogeneity. BioData mining 8: 1-12.
2. Gamadia LE, et al. (2001) Differentiation of cytomegalovirus-
specific CD8+ T cells in healthy and immunosuppressed virus
carriers. Blood 98: 754-761.
3. Lanzavecchia A, F Sallusto (2002) Progressive differentiation
and selection of the fittest in the immune response. Nature
Reviews Immunology 2: 982-987.
4. Seder RA, PA Darrah, M Roederer (2008) T-cell quality in
memory and protection: implications for vaccine design.
Nature Reviews Immunology 8: 247-258.
5. Kaech SM, EJ Wherry (2007) Heterogeneity and cell-fate
decisions in effector and memory CD8+ T cell differentiation
during viral infection. Immunity 27: 393-405.
6. Milush JM, et al. (2009) Functionally distinct subsets of
human NK cells and monocyte/DC-like cells identified by
coexpression of CD56, CD7, and CD4. Blood 114: 4823-
4831.
7. Lanier L, et al. (1986) Natural killer cells: definition of a
cell type rather than a function. The Journal of Immunology
137: 2735-2739.
8. Yang ZZ, SM Ansell (2012) The tumor microenvironment
in follicular lymphoma. Clinical Advances In Hematology
& Oncology: H&O 10: 810-818.
9. Cooper MA, TA Fehniger, MA (2001) Caligiuri The biology
of human natural killer-cell subsets. Trends in immunology
22: 633-640.
10. Senpuku H, et al. (2016) CD56 dim CD16 high and CD56
bright CD16- cell percentages associated with maximum
knee extensor strength and incidence of death in elderly.
SpringerPlus 5: 1-11.
11. Dulphy N, et al. (2008) An unusual CD56brightCD16low NK
cell subset dominates the early posttransplant period following
HLA-matched hematopoietic stem cell transplantation. The
Journal of Immunology 181: 2227-2237.
12. André P, et al. (2000) Modification of P-selectin glycoprotein
ligand-1 with a natural killer cell-restricted sulfated
lactosamine creates an alternate ligand for L-selectin.
Proceedings of the National Academy of Sciences 97: 3400-
3405.
13. Solal-Céligny P, et al. (2004) Follicular lymphoma
international prognostic index. Blood 104: 1258-1265.
14. Linn BS, MW LINN, L Gurel (1968) Cumulative illness rating
scale. Journal of the American Geriatrics Society 16: 622-626.
15. Pawelec G, et al. (2005) Human immunosenescence: is it
infectious? Immunological reviews 205: 257-268.
16. Derhovanessian E, et al. (2011) Infection with cytomegalovirus
but not herpes simplex virus induces the accumulation of
latedifferentiated CD4 + and CD8 + T-cells in humans. Journal
of General Virology 92: 2746-2756.
17. Koch S, et al. (2008) Multiparameter flow cytometric analysis
of CD4 and CD8 T cell subsets in young and old people.
Immunity & Ageing 5: 1-12.
18. Woodbury N (1996) Department of Immunology, Institute
of Cancer Research, Health Enterprise Rikshospitalet-
Radiumhospitalet, Oslo, Norway; 2Department of Radiation
Biology, Institute of Cancer Research, Health Enterprise
Rikshospitalet-Radiumhospitalet. Blood 88: 1437-1444.
19. Liao CM, MI Zimmer, CR Wang (2013) The Functions of Type
I and Type II Natural Killer T (NKT) Cells in Inflammatory
Bowel Diseases. Inflammatory bowel diseases 19: 1330-
1338.
20. Terabe M, JA Berzofsky (2008) The role of NKT cells in
tumor immunity. Advances in cancer research 101: 277-348.
21. Dhodapkar MV, et al. (2003) A reversible defect in natural
killer T cell function characterizes the progression of
premalignant to malignant multiple myeloma. The Journal
of experimental medicine 197: 1667-1676.
22. Giaccone G, et al. (2002) A phase I study of the natural killer
T-cell ligand α-galactosylceramide (KRN7000) in patients
with solid tumors. Clinical Cancer Research 8: 3702-3709.
23. Molling JW, et al. (2007) Low levels of circulating invariant
natural killer T cells predict poor clinical outcome in patients
with head and neck squamous cell carcinoma. Journal of
Clinical Oncology 25: 862-868.
24. Pennock ND, et al. (2013) T cell responses: naive to memory
and everything in between. Advances in physiology education
37: 273-283.
25. Klenerman P, A Oxenius (2016) T cell responses to
cytomegalovirus. Nature Reviews Immunology 16: 367-377.
26. Reinke S, et al. (2013) Terminally differentiated CD8+ T
cells negatively affect bone regeneration in humans. Science
translational medicine 5: 177ra36-177ra36.
27. Yap M, et al. (2014) Expansion of highly differentiated
cytotoxic terminally differentiated effector memory CD8+
T cells in a subset of clinically stable kidney transplant
recipients: A potential marker for late graft dysfunction.
Journal of the American Society of Nephrology 25: 1856-
1868.
28. D’Asaro M, et al. (2006) Increase of CCR7- CD45RA+
CD8 T cells (TEMRA) in chronic graft-versus-host disease.
Leukemia 20: 545-547.
29. Betjes MG, et al. (2012) Terminally differentiated CD8+
Temra cells are associated with the risk for acute kidney
allograft rejection. Transplantation 94: 63-69.
30. Matteucci E, et al. (2011) Altered proportions of naive, central
memory and terminally differentiated central memory subsets
among CD4+ and CD8+ T cells expressing CD26 in patients
with type 1 diabetes. Journal of clinical immunology 31:
977-984.
31. Lugos MD, The Effect of Cytomegalovirus infection on
Follicular Lymphoma biology. 2017, University of Liverpool.
