Author(s): <p>Shivshankar Timmanpyati, Aarti Bhillare*, Aravind Krishnamurthy, Divya Choudhary, Sunitha Premlatha, Prasanthi Suryanarayana V, Rajeshwari, Richa Shukla, Dedeepiya Devaprasad, Esther Sathiaraj and Mini N</p>
Sarcopenia is a widely prevalent skeletal muscle condition that progresses over time and is associated with adverse outcomes. It is characterized by an accelerated loss of muscle mass and functional ability. Proposed causes of sarcopenia include aging, physical inactivity, malnutrition, hormonal disturbances, inflammation, and neurodegenerative changes. According to the IAPEN INDIA (India Association for Parenteral and Enteral Nutrition), nutritional management should be integrated into the diagnostic process for all chronic diseases, including cancer. Studies recommend aggressive nutritional management, providing an energy intake of >30 kcal/kg/day and protein intake of >1.2g/kg/day for patients with sarcopenia. Published literature suggests that adequate intake of antioxidants, such as vitamin D, E, and C, along with Omega-3 fatty acids, creatine, and L-carnitine, might be beneficial for preserving and improving sarcopenia, and functional outcomes. In summary, nutritional management complemented with resistance exercise, under strict supervision, is key to preventing and treating sarcopenia in cancer patients.
Sarcopenia was defined by the European Working Group on Sarcopenia in Older People (EWGSOP) in 2010. Later, they included sarcopenia defining specific cut-off points in 2019. Sarcopenia is also referred to as ‘myopenia’ and is currently defined as ‘progressive and generalized loss of skeletal muscle mass (SMM) leading to low physical performance’. This condition is associated with adverse outcomes that include frailty, falls, functional decline, and even mortality. Sarcopenia has emerged as a critical concern in the realm of oncology, profoundly impacting the prognosis and quality of life (QoL), and is recognized as a vital prognostic factor for advanced cancer patients. It is consistently associated with disruptions in amino acid metabolism, characterized by an imbalance that causes muscle protein breakdown over synthesis, resulting in the reduction of muscle fibers. Categorized into primary and secondary sarcopenia, the former is associated with the natural aging process, while the latter with chronic inflammation mediated by cytokines, as observed in cancer. Unlike in aging, where the loss of muscle mass is a natural process, sarcopenia in cancer patients represents the degradation of normal body tissue function in response to systemic inflammation, owing to a host-tumor interaction [1-4].
Experts aim to explore the intricate relationship between sarcopenia and cancer, elucidating its prevalence, underlying mechanisms, and nutritional management, with a focus on clinical implications. As a complex condition, sarcopenia poses challenges in the management of cancer patients and significantly influences treatment outcomes. Understanding the complexities of this association is imperative for developing targeted interventions that enhance patient well-being and overall survival.
The prevalence of sarcopenia varied across different patient groups. Researchers reported a 29% prevalence of sarcopenia in the older population. Oncological patients from the Western population indicate a higher incidence of sarcopenia prevalence. However, data on the Indian population are scarce.
The available data from India indicate a prevalence between 31% to 90%. A meta-analysis investigating sarcopenia prevalence in various solid tumors revealed an overall occurrence of 35.3%. Certain cancers, including esophageal, urothelial, cholangiocarcinoma, prostate, sarcomas, and thyroid cancers, exhibited a prevalence exceeding 50%. The study highlighted variations in sarcopenia prevalence across different tumor types and oncologic settings, with a higher incidence in palliative (49.2%) compared with curative settings (39.6%). Sixteen percent of breast cancer survivors were diagnosed with sarcopenia, which more commonly included post-menopausal women. It was observed that 40.3% of patients who underwent curative hepatectomy for hepatocellular carcinoma were sarcopenic. Similarly, 33% cholangiocarcinoma patients who underwent major hepatectomy with extrahepatic bile duct resection, were sarcopenic. In advanced lung cancer patients receiving palliative chemotherapy, the prevalence of sarcopenia, determined by skeletal muscle cross-sectional area (SMCA) at the third lumbar vertebrae, was observed to be as high as 71%. Sarcopenia is more common among women, people with lower body mass index (BMI), and those with liver dysfunction. A systematic review of 867 lung cancer patients found significant skeletal muscle loss with a standardized mean difference (SMD) of: -0.25 (95% CI -0.47 to -0.03). The prevalence of sarcopenia in the pre-treatment phase varied across studies from 35% to 74%. One study reported prevalence of sarcopenia both before and after chemotherapy, showing an increase from 35% to 59% [5-7].
Sarcopenia and cachexia are distinct conditions. The term “cachexia” originates from the Greek words “kakòs” (bad) and “héxis” (condition). Cancer cachexia is a multifactorial syndrome characterized by progressive SMM loss and the additional burden of adipose tissue wastage. Cancer cachexia and sarcopenia are closely related overlapping conditions, which are generally neglected, underdiagnosed, and undertreated. In cancer patients, systemic inflammation and other metabolic abnormalities commonly result in functional impairment, causing complications, reducing patients’ quality of life (QoL), and worsening disease outcomes. Patients with metastasis experience a higher incidence of cancer cachexia. Treatment for this condition is primarily confined to systemic therapy, with the goal of palliative care focusing on prolonging survival while maintaining a good QoL. Owing to differences in diagnostic criteria and terminology, it is challenging to determine the exact prevalence of obesity and sarcopenia in cancer patients [8,9].
In many cancers, sarcopenia has served as a prognostic factor for disease progression and survival. A retrospective study by Harimoto et al. found that the five-year recurrence-free and overall survival rate was significantly lower in sarcopenic patients. Similar results were observed in advanced breast and lung cancer patients. Sarcopenia may interfere with cancer patients’ treatment outcomes. Its prevalence in advanced cancer patients varies across different types of cancer and disease stages; it is also influenced by the choice of measuring tools. Sarcopenia is increasingly recognized as a poor prognostic factor. It is now acknowledged that chemotherapy and specific targeted agents cause sarcopenia, resulting in reduced physical functionality and QoL. Pre-treatment sarcopenia predicts chemotherapy toxicity, poor response, increased disability, diminished anti-tumor response, and survival. A study of advanced solid and hematologic malignancies in elderly patients indicated that sarcopenia and chemotherapy toxicity are significant concerns for this group. Early detection and treatment of sarcopenia-cachexia-malnutrition are crucial for cancer patients to prevent the deterioration of physical activity (PA) and to manage, maintain, and improve their functional capacity, mental well-being, and QoL [7,10-12].
Aging causes a natural imbalance between anabolic and catabolic processes, leading to alterations in skeletal muscle homeostasis, which is proposed as the primary mechanism for the development of sarcopenia. Sarcopenia can also arise from chronic inflammatory conditions, hormonal disturbances, diabetes, vascular changes, as well as serious issues related to renal, pulmonary, and cardiac failure, along with immobility. In sarcopenic muscles, there is a decline in the size and number of cells, especially in type II muscle fibers, accompanied by intramuscular and intermuscular fat infiltration. Additionally, there is a reduction in satellite cell numbers and functions, whose primary role is to replace and repair damaged muscle fibers. These reduction in functions might result from alterations of systemic factors responsible for activities and differentiation, such as muscle stem cell niche factors, myogenin, and transforming growth factor-beta (TGF-β). TGF-β, neuromuscular junction dysfunction, inflammation, insulin resistance, decreased numbers of motor units, mitochondrial dysfunctions, oxidative stress, myostatin, and bone morphogenetic proteins contribute to muscle loss. Denervation of single muscle fibers also causes reduction in type II fibers, which get replaced by type I fibers and fat tissue [13-17].
Early diagnosis of sarcopenia in cancer is crucial for targeted intervention, as it can adversely impact treatment outcomes and overall QoL. European Working Group on Sarcopenia in Older People 2 (EWGSOP2) and the Asian Working Group for Sarcopenia (AWGS) have proposed an algorithm for sarcopenia detection known as the Find-Assess-Confirm-Severity (F-A-C-S) approach, which follows a sequential pathway and structured diagnostic approach (Table 1). For example, if sarcopenia screening indicates a positive result in the initial step for muscle strength [hand grip strength (HGS)], it must be assessed for SMM. A decrease in SMM confirms the sarcopenia. Further assessment through physical performance tests will diagnose the severity of the condition, with muscle strength emerging as the most dependable indicator of muscle function [18].
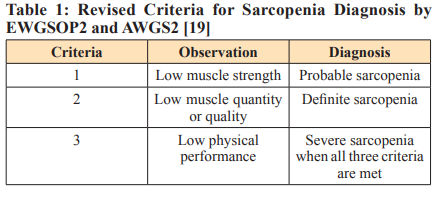
The SARC-F screening tool and the Ishii screening tool are recommended by EWGSOP2 for diagnosing sarcopenia.
The SARC-F is a self-administered, rapid diagnostic screening tool. It comprises five elements: muscular strength, walking support, ability to stand up from a chair, climbing stairs, and incidents of falls. Each aspect is assigned a score ranging from 0 to 2, resulting in a cumulative score that spans from 0 to 10. A lower score indicates better performance, while a higher score reflects increased difficulty. Notably, a score of four or above demonstrates high sensitivity and specificity in identifying sarcopenia, and is indicative of unfavourable future outcomes. The sensitivity of SARC-F is low to moderate; it possesses a high specificity to anticipate low muscle strength [20,21].
The Ishii screening tool demonstrates a high level of sensitivity and specificity. It is a composite score of age, HGS, and calf circumference (CC) with a potential score range of 0 to 145 for men and 0 to 150 for women. The cut-off values for determining sarcopenia are set at 105 for men and 120 for women. However, there are different screening tools for sarcopenia.
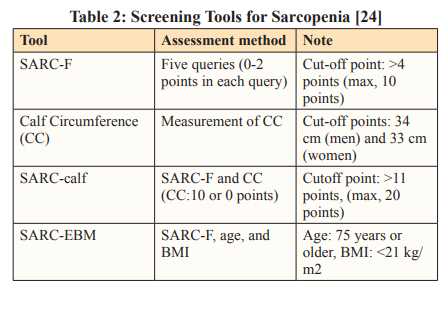
For frailty, the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) Expert Working Group (2022) recommended screening all patients above the age of 70 years using a validated tool such as the Clinical Frailty Scale (CFS), Hospital Frailty Risk Score, the FRAIL scale, or the Frailty Index, along with the Edmonton Frail Scale. Those patients who screen positive for frailty should undergo further clinical assessment. The recommended tools are the Frailty Phenotype, Frailty Index, or data collected from a Comprehensive Geriatric Assessment (CGA). Screening instruments for assessing frailty comprise the FRAIL scale and the Edmonton Frail Scale. A list of sarcopenia screening tools has been listed in Table 2 [22,23].

Hand grip strength (HGS): This was assessed using the handheld dynamometer. According to the AWGS (2019), sarcopenia could be indicated if the HGS falls below 28 kg for men or 18 kg for women. HGS indicates a strong correlation with other muscle compartments.
Chair stand test: According to the AWGS (2019), if completing five rises takes >12 s, it serves as another criterion for identifying sarcopenia. During this test, individuals are required to rise five times as quickly as possible with their arms folded. It is important to note that mobility issues can impact performance on this test, making it a relevant consideration in the assessment [25].
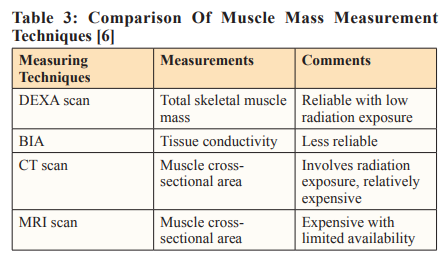
Various techniques, such as dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), and computed tomography (CT) or magnetic resonance imaging (MRI) can assess muscle mass. Muscle volume is represented as total body SMM, appendicular skeletal muscle mass (ASM), or the cross-sectional area of specific muscle groups.
BIA, a cost-effective and radiation-free tool, could detect sarcopenia in cancer patients before treatment, offering a practical alternative to CT, DEXA, and MRI [26].
Standard anthropometric measurements, such as Mid-Upper Arm Circumference (MUAC), Mid-Arm Muscle Circumference (MAMC), or CC lack general international standardization and reference values tailored to diverse patient demographics. Despite these limitations, they can complement more sophisticated measures. Their affordability, ease of execution, and widespread availability make them viable options in various healthcare settings [27].
The accuracy of ultrasound tests for diagnosing sarcopenia varies and ranges from low to moderate. It depends on factors such as ultrasound parameters, the muscles being measured, the reference standards used for comparison, and the characteristics of the individuals. Combining indicators of both muscle quality and quantity might enhance the accuracy of the diagnostic test and improve the overall precision of sarcopenia diagnosis when using ultrasound as a diagnostic tool [28].
Low CC is not directly indicative of low Skeletal Muscle Index (SMI) in cancer patients, but it can serve as an alternative, non- invasive, and easily accessible method for preliminary screening. However, it can predict performance and survival in older individuals, particularly when the cutoff is set at < 31 cm, and offer a convenient way to identify cancer patients with potentially adequate SMI [29].
Individuals with large body sizes possess greater muscle mass. Therefore, when measuring muscle mass, adjustments for body size are essential (Table 3). This can be achieved through height squared (ASM/height²), weight (ASM/weight), or BMI (ASM/ BMI) [30].
Muscle performance tests such as the Short Physical Performance Battery (SPPB), gait speed measurement, and the 400-meter walk test, assess overall body function, particularly mobility and transfer abilities. The SPPB, which combines balance, gait speed, and chair stand tests, provides a score between 0 and 12, with a score of ≤ 8 indicating low performance. Gait speed, measured over a 4-meter distance, has a cut-off at < 0.8 m/s. However, the brief distance may impact evaluation if there are issues in the gait initiation phase. The 400-meter walk test assesses both physical function and endurance by completing 20 laps of a 20-meter distance in the shortest time possible. All these tests can be conducted without additional equipment; their utility is limited in individuals with cognitive impairment, physical disability, or gait and balance disorders [5,31].
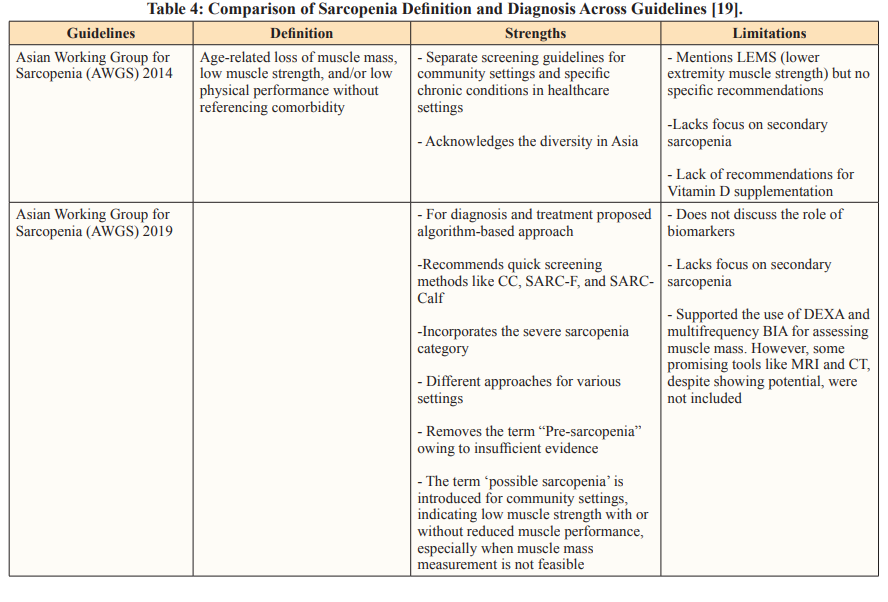
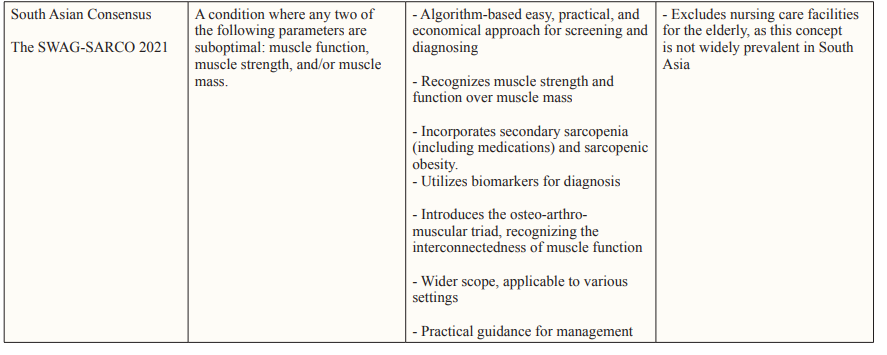
Adapting Sarcopenia Diagnosis with Asian Perspectives Using Western diagnostic criteria for assessing health conditions in Asian individuals may not be entirely appropriate. Special considerations are necessary owing to inherent physiological differences (Table 4). Asians typically have smaller body sizes, higher levels of adipose (fat) tissue, and are more physically active than their Western counterparts. In response to these distinctions, the AWGS is actively working toward establishing a consensus for diagnosing sarcopenia. This involves systematically gathering evidence from research studies specific to sarcopenia in Asian populations [25].
L-Carnitine: Patients with cancer cachexia exhibit reduced levels of carnitine. Carnitine levels were low in gastrointestinal cancer patients suggesting it as a biomarker of sarcopenia and nutritional status [32].
Creatinine is the byproduct of creatine metabolism, predominantly found in muscle tissue. Serum creatinine levels can be used as a biomarker which could indicate the state of the skeletal muscles. Approximately 1.9 kgs of skeletal muscle produce 1 mmol of creatinine in urine. Nonetheless, patients with cancer cachexia have observed a decrease in serum creatine levels.
Uric acid: Oxidative stress induces muscle loss owing to physical inactivity and sarcopenia. Uric acid acts as an antioxidant, protecting against the damages by free radicals. Increased uric acid levels improve HGS and muscle function in older adults. Uric acid levels are influenced by foods such as red meat, fish, and alcohol [33-36].
Urinary Titin N-terminal Fragment Concentration (UTF): Increased UTF is found in the urine of sarcopenic patients indicating it as a useful biomarker. Preoperative nutritional assessment of gastrointestinal tract and hepatobiliary pancreatic cancer patients has found UTF to be significantly higher in sarcopenic patients (P = 0.04), and indicated negative correlations with skeletal muscle volume index (r = -0.16, P = 0.04) [37].
Leptin and inflammatory markers: Leptin is secreted by adipose tissue and increases during overnutrition and obesity. Increased leptin production leads to pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-12 (IL-12) that initiate catabolic processes within muscles. These cytokines have been proposed as one of the pathophysiological mechanisms that promotes sarcopenia. Similar associations were found for elevated levels of inflammatory markers such as CRP, IL-6, and sIL-6r. Central obesity also promotes inflammation [38,39].
3-Methylhistidine (3-MH): 3-MH is an amino acid primarily found in skeletal muscle. Muscle protein degradation and 3-MH excretion in urine can be used to estimate the rate of skeletal muscle degradation which can represent a potential biomarker for muscle protein turnover. This can help to diagnose sarcopenia and frailty. Foods such as meat and fish can influence plasma concentrations of 3-MH. 3-MH is also released in urine by sources other than skeletal muscles, and medications can influence the presence of 3-MH in the urine [40].
Antioxidants: It is postulated that low levels of antioxidants, such as glutathione peroxidase and thioredoxin, along with exogenous antioxidants may attenuate the impact of reactive oxygen species (ROS). Low selenium levels have been related to low muscle mass in the elderly [41].
Visceral Proteins and Insulin-like Growth Factor (IGF-1): Malnutrition is also reflected in serum protein levels such as prealbumin, albumin, transferrin, and retinol-binding protein. IGF-1 is primarily produced in the liver. It is involved in various anabolic processes within the skeletal muscle. Low levels of IGF-1 concentrations are associated with skeletal muscle atrophy, indicating its potential relationship with the development of sarcopenia [42].
Owing to the prognostic significance of sarcopenia in cancer patients, early detection and intervention are clearly imperative. Aerobic exercise, strength training, nutrient supplementation, and rehabilitation collectively help in the effective nutritional management of sarcopenia. The goal is to prevent further muscle loss and enhance functional status. Early intervention could possibly reduce or prevent falls and disabilities, and might enhance physical activities, prevent hospitalization, improve QoL, and reduce mortality in the affected patients.
Diminished nutrition can contribute to sarcopenia development and progression. Studies indicate an association between sarcopenia and low protein and low levels of vitamin D vitamin C, B6 (pyridoxine), and B12 (cobalamin). A well-planned diet, rather than individual nutrient supplementation, may be more effective in preserving muscle mass and improving physical function [43-46].
There is limited research available on nutritional recommendations for sarcopenia management in cancer patients. Ayano et al. (2020) found that in multivariable models, after adjusting for confounding factors, an energy intake of ≥30 kcal/kg/day (p = 0.011) along with a protein intake of ≥1.2 g/kg/day based on the ideal body weight (IBW) exhibited improvement in tongue strength post- treatment (p = 0.020). To prevent further loss of muscle mass and maintain muscle quality and physical independence, an adequate amount of calorie intake is necessary. The energy requirements of an individual comprise the total of the resting energy expenditure (REE), PA, and diet-induced thermogenesis. The gold standard for determining REE is by indirect calorimetry. IAPEN INDIA, following similar guidelines of European Society for Nutrition and Metabolism (ESPEN) 2021, recommends energy requirements for cancer patients to be similar to healthy subjects, typically ranging between 25 - 30 kcal/kg/day [47-49].
Dietary protein serves as the primary physiological stimulus for muscle protein synthesis, promoting muscle mass, and reducing muscle loss.
The ubiquitin-proteasome system (UPS) contributes to cellular protein degradation, while protein or amino acid nutritional support can downregulate MuRF-1 and Atrogin-1 levels, key regulators of ubiquitin-proteasome pathway (UPP), thereby ameliorating sarcopenia [50].
Research has indicated sarcopenic cancer patients with an average protein intake of < 1.2 g/kg exhibited muscle wasting, whereas those consuming > 1.4 g/kg, maintained muscle mass throughout the treatment. In another study of 95 sarcopenic older patients, a protein intake of ≥1.2 g/kg/day based on the IBW indicated a significant positive association with increased tongue strength (p= 0.020) [49,51,52].
Despite recommendations by ESPEN (2021) and IAPEN INDIA (2021) for protein intake of up to 1.5 g/kg/day for cancer patients, specific amino acids such as branched-chain amino acids, especially leucine and lysine, have shown a strong association with increase in muscle protein synthesis through up-regulation of mTORC1 activity. Leucine, which promotes anabolic activity and regulation of protein synthesis, has shown improvement in skeletal muscle myofibrillar protein synthesis (MyoPS) after resistance exercise, on a 3 g and 3.5 g supplementation in younger and older men, respectively. Studies suggest that hydroxy methyl butyrate (HMB) may play a beneficial role in maintaining and building muscle mass, with randomized control trials indicating its positive impact in cancer patients and older adults. Further well-designed and high-quality trials are required to comprehensively explore broader clinical benefits of leucine and HMB supplementation [36,53-58].
Out of the total free amino acid pool, skeletal muscle comprises approximately 50–60% glutamine. It is the most synthesized amino acid in the muscle. Additionally, amino acid supplementations such as glutamine and L-arginine have increased in lean body weight. Another study involving 44 patients undergoing surgery for non-metastatic head and neck cancer, randomized half of the sample to consume glutamine supplementation (0.3 g/kg/ day) for four weeks, while the other half received a placebo. The study, conducted in 44 head and neck cancer patients, showed an increase in lean mass in all patients of the intervention group post-operatively [52,59,60].
Patients with cancer cachexia exhibit reduced levels of carnitine. Few studies have shown an increase in body weight, PA, lean body mass, and QOL with 4 g of carnitine supplementation, and reduced fatigue with 6 g of supplementation. Larger studies are warranted to confirm the clinical outcomes [45,61].
Patients with cancer cachexia may be creatine-deficient. Creatine is produced in the liver and stored in the skeletal muscle, from where it acts as an instant fuel reserve for energy generation during muscle contraction. Creatine supplementation, along with physical exercise, can be an effective dietary strategy in the management of conditions like sarcopenia and muscle loss. Creatine supplementation, along with resistance training, significantly improves aging muscle mass, upper body strength, bone mineral density, and indices of bone biology [34,62,63].
Vitamin-D deficiency induces biological processes of sarcopenic muscle atrophy. Vitamin-D deficiency significantly diminishes gastrocnemius muscle mass, muscle fiber cross-sectional area, and grip strength. In a recent review, it was concluded that muscle strength and physical performance were significantly impacted by vitamin D, particularly in women with low baseline values (< 25 nmol/l) [64,65].
ROS may lead to oxidative muscle damage and mitochondrial dysfunction during muscle aging. Antioxidants, such as vitamins C and E, prevent oxidative stress owing to ROS activity and might play a vital role in treating sarcopenia. However, there is limited evidence to state that vitamins C and E can prevent or treat sarcopenia [64].
Omega-3 fatty acids are unsaturated fatty acids, namely α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). The inflammatory process and cytokine activity lead to skeletal muscle atrophy, cell death, reorganization of existing tissues, and activation of endoplasmic reticulum stress (ERS) and autophagy. Omega-3 fatty acids have the potential to attenuate ERS and autophagy occurring in skeletal muscles that are undergoing atrophy. DHA supports mitochondrial biogenesis and remodeling of skeletal muscle fiber, and prevents or delays muscle wasting by inhibiting degradation of muscle proteins [66-68].
Nitrate is found in skeletal muscle tissues where it gets metabolized. Nitrate produces nitric oxide during exercise, and improves muscle contraction. Few studies have found that a higher nitrate intake is associated with improved grip strength, faster runs, stronger knee extension, faster timed starts, and increased muscle strength [64].
Prebiotics promote the growth of beneficial bacteria. Probiotics promote the production of some amino acids that regulate skeletal muscle activity by improving insulin sensitivity. Alterations in the gut microbiota reduce the production of beneficial metabolites such as short chain fatty acids, and could lead to intestinal leakage of bacterial endotoxins into the peripheral blood, causing systemic inflammation. This is associated with aging and muscle wasting.
Supplementation of probiotics can limit inflammation and oxygen stress [64].
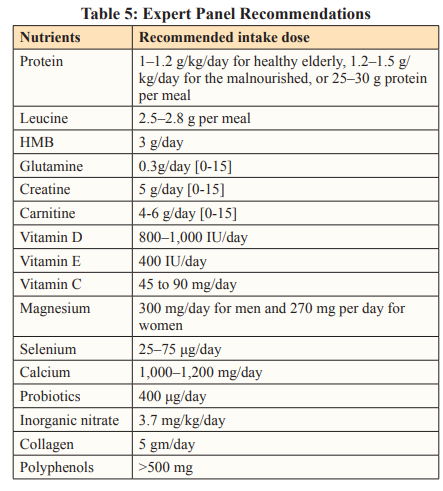
Inadequate intake of micronutrients, such as calcium, magnesium, sodium, and selenium, is associated with an increased risk of sarcopenia. Magnesium, selenium, and calcium, are the most promising minerals for the effective prevention or treatment of sarcopenia. Table 5 summarises the macro and micro nutrient requirements to address sarcopenia management.
Magnesium is involved in maintaining muscle mass and protecting muscle tissue from oxidative damage. Studies have indicated a positive association between higher magnesium intake and SMM, explosive leg strength index, and greater grip strength, suggesting magnesium’s ability to improve sarcopenia.
Patients with selenium deficiency develop skeletal muscle disease, characterized by fatigue, muscle pain, weakness of proximal limb, and increased serum creatine kinase. Studies indicate that selenium concentrations were negatively associated with physical function [69,70].
Collagen accounts for one-third of the total protein in the human body, and is the most abundant form of structural protein in the body. It contributes about 65%−80% of tendon dry weight. Extramyocellular connective tissue regulates tendon contractility. Collagen is a key component of extracellular connective tissue and is essential for the strength, regulation, and regeneration of the tissue. Collagen peptides or gelatin, may help stimulate muscle connective tissue synthesis, thereby potentially improving sarcopenia [64].
Polyphenols have antioxidant and anti-inflammatory properties. They increase protein synthesis by activating the Akt/mTOR pathway. Polyphenols regulate the expression of miRNAs and IGF-1 signalling pathway. Further research in this domain remains inconclusive and unexplored [64].
Adapted from: Liu et al.’s ‘Advances in nutritional supplementation for sarcopenia management.’ Compiled from Front Nutr. 2023 and other sources.
In a recent review testosterone supplementation in men with low serum levels (< 200-300 ng/dl), concluded improved effects on muscle mass and modest to minimum benefit on muscle strength and physical performance, respectively. Minimal and few incidences of adverse events were reported in the studies [65].
To address sarcopenia, a strategy integrating resistance training with nutritional intervention is crucial. Several studies have demonstrated that supervised resistance exercise helps prevent and treat sarcopenia in cancer patients. For instance, a review by found that resistance exercise improved muscle mass by 0.9 kg higher than the control group. Various types of exercises including aerobics, resistance training, balance training, and weight training have proved beneficial. In another study, low or moderately intense and low weight-bearing exercises have significantly increased SMM, leg strength, and grip strength. Studies on exercise recommendations remain limited and heterogenous, necessitating further evidence of comparative population studies [71,72].
Water is required to transport nutrients to all cells and transport waste out of the body. Muscles primarily consist of water, comprising almost 76% of the muscle mass. Dehydration can affect both the mechanical and metabolic functions of muscles, leading to issues such as muscle contraction problems, cramps, impaired muscle tone, electrolyte imbalance, and frailty. A well hydrated body prevents muscle cramping and improves muscle functionality, such as contractions and faster movements. Thus, intracellular water in lean mass is a good indicator of muscle quality and cell hydration. Currently, there are no universally accepted recommendations on the optimal daily water intake for individuals [73-75].
Integrating sarcopenia screening and assessment into the care of cancer patients, along with early nutritional intervention, in treatment algorithms, can help prevent treatment complications. Sarcopenia assessments can help predict treatment outcomes and serve as a valuable biomarker. Timely and comprehensive nutritional strategies, coupled with exercise training under strict supervision, can help overcome complications. However, further high-quality research is required to explore additional management options and multimodal therapies to achieve the goals of nutrition rehabilitation.
This study has been funded by Nutri Health Foundation. H.No.7- 1-45/1, Near Kotak School Kirlampudi Layout, LB Colony Visakhapatnam, AP, India, 530017. Code for Article: NHF/2023- 24/06.
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
The authors have not been paid to write this article by any pharmaceutical company or other agency. The authors were not precluded from accessing data in the study, and they accept responsibility for submitting it for publication.
Conflict of Interest: None
Mr. Shivshankar Timmanpyati and Mrs. Aarti Bhillare reviewed and wrote the paper. They have directly accessed and verified the underlying data reported in the manuscript. They had full access to all the data in the study and accept responsibility to submit for publication. The co-authors have been actively involved in discussions and co-reviewing the paper.
I would like to express my sincere gratitude to the following individuals for their invaluable contributions to this study: Mr. Shivshankar Timmanpyati, a Chief dietician with Tata Memorial Hospital, for reviewing and writing; Mrs Aarti Bhillare, a Dietician, for reviewing and writing; Dr. Aravind Krishnamurthy, a Professor and Head of Surgical Oncology at Adayar Cancer Institute, Chennai, for discussing and co-reviewing; Ms. Divya Chowdary, Chief dietician from Rajiv Gandhi Cancer Hospital, Delhi, for discussing and co-reviewing; Ms. Sunitha Premlatha, a Chief dietician at Yashoda Hospitals, Hyderabad, for discussing and co-reviewing; Ms. Prasanthi Suryanarayana V, a Chief dietician at Basavatarakam Indo American Cancer Hospital, for discussing and co-reviewing; Ms. Rajeshwari, a Chief clinical dietician at Apollo cancer Specialty Hospitals, Chennai, discussing and co-reviewing; Ms. Richa Shukla, HOD Clinical Nutrition at Jehangir Hospital, Pune, for discussing and co-reviewing; Dr. Dedeepiya Devaprasad, HOD Critical Care at Apollo Cancer Specialty Hospitals, Chennai for discussing and co-reviewing; Dr. Esther Sathiaraj, a Chief dietician at HCG hospitals, Bangalore, for discussing and co- reviewing; Ms. Mini N, Chief dietician at RCC Hospital, Kerala, for discussing and co-reviewing.
Search Strategy and Selection Criteria: References for this Review were identified through searches of PubMed with the search terms “sarcopenia”, “muscle mass”, “nutrition”, and “exercise” from 1989 until 2023. Articles were also identified through searches of the authors’ files. Only papers published in English were reviewed. The final reference list was generated based on originality and relevance to the broad scope of this review.
