Author(s): Hit Kishore Goswami
Heterosporous sporangia discovered in Isoetes pantii Goswami & Arya are known as heterosporangia. Heterosporangia produce thousands of alete, monolete and trilete microspores and a few dozen megaspores. This trait is inherent within the genome of I pantii because plants collected from a location in South Gujarat also exhibit this feature. Additionally, initial collections and followup studies since 1966 have been consistently showing two types of megaspores within the heterosporangia. While microspores and megaspores develop in each and every hetrosporangium, additional unusual megaspores, variable in shape and size (180-220 micron), do not occur in all heterosporangia of all plants. These spores closely resembling fossil ancestral spores are being now termed as “Chalonospores”. Thus, a living spore genus Chalonospora is being named and defined as a spore discovered in a living plant which recall fossil ancestry of the genus but are never found in any other extant species of the genus. Since Chalanospores also originate from spore mother cells, as other microspores and megaspores do, this indicates that spore mother cells found in heterosporangia are genetically variable in expressions. Quite likely, a few megaspore mother cells must be possessing gene combinations with “revitalized relic DNA sequences”. As different types of chalonospres have been observed, variable gene combinations must be representing sporadic expressions of different lycopods of the vast geological past. Germination studies prove that these spores are endowed with different genetic make-up, as the gametophyte produced on soil culture produce unique single cell rhizoids with spiny outer layer. This apparent genomic reshuffle is related with frequently encountered chromosome breaks, translocations and irregular presence of 2n=36 –to 39 chromosomes in such plants. However, Isoetes pantii plants possess 2n=48 chromosomes with X-Y mechanism. No other species in the world flora of the genus has exhibited sex chromosomal mechanism and possessed n=12 chromosome series. With greater probability this appears plausible to imagine that naturally imposed chromosomal aberrations have rejuvenated genes which might have been regular expressions within the genome of some of the lycopods of Carboniferous and thereafter.
Plant biologists, who have been interested in evolution of plants, particularly among pteridophytes must have realized that the genus Isoetes L has become the most popular plant within past fifty years and this distinction and special recognition has been primarily based on the pioneering work of fossil flora of lycopods and related genera [1-11]. One of the major thrilling experiences has been based on the knowledge of giant ancestors of this small aquatic weed and the greatest evidence of reduction series, which culminated in Isoetes. Gross morphology reminds it to be a “grasslike “but anatomy and reproductive organs are like “conserved pocket edition” of the giant Lepindodendrids etc of the geological past. The discovery of heterosporous sporangia in Isoetes pantii Goswami & Arya [12-14]. which was called as startling discovery in the plant kingdom by many scholars in the relevant field of pteridology (Late Professors DD Pant, WG Chaloner and, Late Mr AC Jermy), a search has been consistently carried out to find variable spores inside the microsporangia and megasporangia of many species of Isoetes.
Interesting morphological, anatomical, chromosomal and biochemical observations have been published but in no other species of the genus, except in I. pantii, the origin and evolution of heterospory within one and the same microsporangium could be ascertained along with additional presence of “ancestral spores” resembling with some trilete megaspores of fossil lycopods [15- 19]. Megaspores from microsporangium had also germinated in soil cultures and a few young sporophytic stages were also encountered thus confirming the sexual viability of spores [29]. Further studies revealed that megaspores and microspores may even germinate to produce respective gametophytes within the intact sporangium; significance of which has been discussed earlier [20,16]. This unique sporangium of Isoetes pantii has been now designated as heterosporangium [20]; this sporangium produces thousands of three types of microspores (large triradiate megaspores and a few highly abnormal megaspores which resemble some fossil lycopods. This has been argued that only within these heterosporangia, certain relic genes have been “rejuvenated” or, revitalized due to genomic reshuffle and epigenetic phenomenon [22-25]. Epigenetic mechanisms have been exclusively operative only within the heterosporangia, as since 1966, not a single megasporangium has been encountered among these plants to contain such a situation. Needless to mention, the development of these unusual megaspores is now an inherent part of the genome of Isoetes pantii; nor even in any species of Isoetes pantii complex which grow in the same ponds [26]. Relevant details have been published elsewhere [15,20,27,28,18,29,25,23].
Initiated in August 1966 at Narsinghgarh (dist Rajgarh, MP, India) has been followed up until 2018 along with visiting as many as 27 places for collections of Isoetes in different states of India for more than five decades. The details of collections, fixations and processing of all parts of the plants as per requirements of study have already been published. Several reviews presenting with detailed morphological, anatomical, chromosomal, biochemical, molecular studies have been added to the botanical literature with the description of a few new species of Isoetes [12- 15,20,27,28,24,26].
Immediately after our report that Isoetes plants bear heterosporous sporangia possessing thousands of microspores and many megaspores within one and the same sporangium the most important question emerged as to whether the megaspores were actually megaspores or only too large spores, and also, whether these could germinate to produce female gametophyte bearing archegonia? [12,13]. Several attempts were made to germinate these spores on several culture media following Kott and Britton (1982) and Taylor et al. (1987) including MS medium with different combinations and additions of enzymes and organic compounds (as per standard protocols of culture media) but no success was achieved [56,38]. However, excellent results were obtained when original soil of the pond was used as the medium for germination studies [29]. Since we also found some abnormal spores (Figs. 1 ABC & 2 A, B) other than usual megaspores with reticulate exine and even sometimes (Fig.2C) joint spore, the same experiment was again repeated after two years. The soil sample was mixed with sterilized distilled water and shaken and repeatedly filtered three or four times. The filtrate, every time was kept in beakers and after two days the supernatant water was discarded and soil was plated in Petriplates. These covered Petriplates containing smooth clay, were wrapped with clean paper and were incubated (dried) at 60 C for a few days. As the fungal microflora is known to be helpful in germination the soil samples were not autoclaved. A heterosporangium was taken and released in to sterilized distilled water in a watch glass and spores were distributed on the surface of the soil layer in four plates. This was ascertained with the help of stereoscopic microscope that there were many large megaspores on the soil fed with sterilized Knops solution. Petriplates were kept at 28-30C for more than a month, but were carefully examined atleast three times in a week. After about 6-7 weeks spores germinated and the observations were still more thrilling (Fig. 4 A-D) [25,16].
Uniform procedure for demonstrations of megaspores as well as observations on chromosomes were adapted. A young heterosporangium on taking out from the plant was placed in 45% acetic acid and then stained with acetocarmine or aceto-orcein (1.5% solution, stored at 15C). Preparations from roots, young sporangia and staining schedules by aceto-orcein were adapted. Also, C-banding procedures of mitotic chromosomes were also attempted following standard protocols and repeated in subsequent studies as well [27,30,19,26].
For detailed observations on spores, a heterosporangium was put in watch glass containing dil. glycerine and gently, spores were released with the help of needles. Spores were examined under low power and on spotting the megaspores, a fine dropper lifted the spore mass on a slide and coverslip was placed. Then spore mass was gradually released by teasing with needles. Slides were prepared and suitable spores were photographed, as has been evident for quite some time [15,16,20,25,27]. SEM studies were performed at Burdwan (West Bengal) University with the help of University Instument centre. Specific details of SEM studies have been described elsewhere. Unfortunately, unusual megaspores did not reveal details of peculiar morphology as has been exhibited in light microscopic studies (Fig 1A B, C & 2A, B, C). A large number of published papers from different authors [31-35]. Were consulted for comparison but such spores have never been described. Figures and comments on these spores are cumulatively presented in the Table.
Genomic DNA was extracted from the plants of Isoetes only after ascertaining that the plant did show clear counts of chromosomes and or the squashes either during mitotic divisions or meiotic divisions observed in young microsporangia, revealed the presence of X and Y chromosomes and microsporangia (now called as heterosporangia) also did clearly show such any of the unusual megaspores (Figs 1 & 2). Genomic DNA extractions and detailed procedures were done by standard protocols [25] [36]. Many experiments were also designed to discover the unique importance of the genomic DNA of Isoetes pantii and comparative approaches were made using genomic DNA samples from other plants wherein, sex chromosomal mechanism has been known for quite some time (eg.Cycas, Ginkgo) Experiments also included human Y chromosome probes particularly PY6, for comparative study with a major outlook to search homology with human Y chromosome; Goswami and Chandorkar, 1994; Bajpai and Goswami, 2002; Goswami 2001, 2004,, 2011, 2012, 2014; Goswami et al, 2006).
Observations on chromosome preparations from roots and young sporangia over these five decades from the same and different localities have repeatedly exhibited numerous evidences for karyoptypic changes and as we now know that unlike other species of the genus Isoetes (Which have chromosome numbers based on n=11: 22, 33, 44, etc) the species constituting Isoetes pantii complex have evolved and possess chromosome numbers in multiples of n=12 [27,19,26]. Series of chromosomal investigations over these years have had suggested that the evolution of new basic chromosome number among the species possessing 2n=36, 48 and 60 chromosomes must have been basically responsible for producing certain plants with odd chromosome numbers (2n= 36- 39 chromosomes) and offering such genic combinations thereby resulting such peculiar spores. Cytological importance of such evolutionary mechanism has been discussed in earlier publications [15,16,20,25,27]. However, this becomes plausible to infer that frequently encountered chromosome breaks, translocations and irregular presence of 2n=36 -to 39 chromosomes in such plants are associated with such revival of “ancestral traits”.
Genomic studies have enabled us in confirming that the plants producing such unusual spores which partly resemble ancestral fossil spores are actually the plants of Isoetes pantii. No other species of Isoetes growing within the same pond and also in other localities has ever shown any such spore nor, any other species has ever possessed a heterosporangium. Detailed phylogenetic studies among different species of Isoetes is in progress.
Most interesting observations on unusual megaspores have been discussed during personal visits to many laboratories and herbaria in India and abroad along with detailed consultations and comparative assessments with many other species of Isoetes described and studied by others [56,37,33-35]. However we know very well that even normal spores of Isoetes have often been compared [15,11,38,18,32]. with many fossil lycopods particularly Isoetites janaianus Banerji Minerosporites Potonie, Valvisporites and even with the Palaeozoic genus Chaloneria and many more spores [11]. But no species of the genus Isoetes in the world flora so far has been known to possess any of these abnormal spores (see Table with Figures) which apparently look like fossil spores of Carboniferous (?) Or any earlier or later geological period. Even among fossil spores described so far, none of the spores presented here is strictly similar. Our long followup observations and each time finding variable shapes of megaspores developing inside the heterosporangia compelled to conclude that in these sporangia, while there are thousands of microspore mother cells which, on meiosis produce three types of microspores, the megaspore mother cells on meiosis produce this unusual megaspores. Obviosly, afew megaspore mother cells must be having random polygenic recombinations and therefore, on meiosis, megaspores with quite different shapes and differing in gross outline and general shape are produced within the same sporangium (Table: Figures 1 A,B,C; 2 A,B.C). There has been a moot question eversince the abnormal spores were found to be comparable with Nikitinosporitis, and then a few spores resembled a Rhaetic genus, Nathorsitsporites Jung as to how, to describe and identify these spores. These spores can even germinate on original locality soil cultures (Fig 4 A-D) [15,20,18,19,39].
Table 1: Comparative Account of Megaspores Tentatively Comparable To Fossil Lycopods Produced Within the Heterosporangia of Isoetes Pantii, As Observed During 1967-2018
Spore & Tentative identity Resemblance with Remarks
|
|
Megaspore more wide than high, upper proximal part is with thick perisporal flaps while lower outer wall is too thick & warty (a part detached to show multilayered thick layers. No spore is known to have dentate projections in wide perispore. The outer warty periphery has been broken so as to reveal many layered exine. |
This type spore was first observed in 1974 and then only in 1982 This spore was found in a sporangium stained with 1.5% aceto-orcein NO COMPARISION |
|
|
A little resemblance with Paxillitrellites cutchhensis Banerji et al 1984. Perispore surrounds entire spore with enlarged flaps on proximal face ; fine hairlike extensions emerge from intine (endospre) , endospore shows waivy spiny layer with no soft zone |
Observed more than four times This spore was found in a sporangium stained with 1.5% aceto-orcein |
|
|
A little resemblance with Paxillitrellites sp. Minerosporites Potonie Trilete megaspore (seen only when dry) but proximal face is covered by two fold wide perisporal spongy flaps. The endospore and outermost perispore have smooth zone (mesospore) all around spore |
Observed many times |
|
|
All megaspores are primarily trilete but this Siamese twin (bodily joint) spore indicates one trilete and another monolete. Stained with dil fast green reticulate striations of exine are prominent . Such spore have very faint outline covering: perispore (perine) layer |
|
|
|
A rarely seen spore with a wavy echinate periphery, tail like structure having broad snout like proximal face with 2to 4 tooth like projections . Wavy warty perispore (perine) on all sides but on proximal face very broad soft perisporal flaps. On drying the spores appeared finely tubeculate |
Observed only twice Resembles none, as of now |
|
|
On account of broader apical neck region May be compared with Isoetites janaianus Banerji |
A rare spore indicating as if lower 1/3rd part filled up with food material |

Figure 3: Isoetes pantii Mitotic chromosomes : (A) 2n=36-38 chromosomes showing translocated fragment (TR) one B and one dot chromosome; stained with 1.5% aceto orcein; (Enlarged-- x 1500) this plant possessed many abnormal megaspores inside the heterosporangia. (B) 2n=48 C banded metaphase showing nearly 2n=46=48 chromosomes and a few breaks; faintly stained euchromatic parts and dark stained heterochromatic large submetacentric X chromosome is clear; many chromosomes show both faint and dark stained regions Breaks are indicated with + and -- signs (X 1500 ; enlarged)
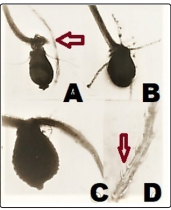
Figure 4: Germination of Unusual Megaspores on Original Soil-Medium (See Text)
Three urn shaped megaspores were found to release young leaves with attached embryonal mass (Arrowed in A) A, B, C: The germinated spores (obviously, gametophyte) A and C appear to be the same type resembling Fig 2 C shows horizontal sporophte at the upper end). Young sporophyte develops (D) spiny growths on the outer face have echinate spiny surface. exine possessing younger embryonal mass and as expected the rhizoids emerg from top of the upper Part; these are unicellular but of two types; smooth walled as well as some spores have spiny projections (arrowed in D).
As many as ten (10) types of megaspores have been identified during past fifty years. However, since all spores maintain some common features, spores are designated or classified by a common generic name Chalanospora keeping an inherent desire to honour late Professor W G Chaloner (London) who was of great help in examining the material, suggesting improvements and validating my observations, ofcourse at the personal level.
A living spore genus Chalonospora is being named and defined as a spore discovered in a living plant which recall fossil ancestry of the genus but are never found in any other extant species of the genus. Such variable spores are grouped under Chalonospores”. These megaspores resembling fossil lycopods born inside the heterosporangia of Isoetes pantii are often round, oval or elliptical, triradiate, rarely monolete and bilateral, measure 184 to 260 mu (micron) in diam; exine has three layers and in almost all spores the proximal face has very broad spongy layer perine (perispore), sometimes in many folds; wide perispore always has either fine traversing tubules or fine spines or even dentate projections. Outer margin all around spore is warty with wavy layer. Dry spores reveal few tubercles, but often with striated perine. SEM pictures of many spores were not able to offer detailed exine ornamentations except some tubercles and rough exine covered the surface. A few spores have also seen to have papillae.
Since frequent prevalence of any one kind of such an unusual spore has not been recorded, no “Holotype” can be assigned, therefore this can be only considered as a tentative spore genus, which represents a spore resembling any of the fossil ancestor (s) abruptly produced within a sporangium of a species but is not present any other extant species of the genus so far known.
As mentioned above, a large number of dispersed fossil flora of megaspores have been found resembling the normal megaspores of the extant genus Isoetes [15,40,38,41,42]. Megaspores of Pleuromeia have also been found to correspond with the morphology of Isoetes megaspores. Their ultrastructural similarity appeared to be of great phylogenetical interest because it had suggested close relationships between the Triassic Pleuromeia and the extant Isoetes. Lugardon and associates in their monumental work have discovered several species of Pleuromeia, a very important Triassic genus testifying reduction series of evolution among lycopods, to have produced spores very much similar to the extant genus Isoetes Papers presenting ultrastructural study of the megaspores in Pleuromeia rossica Neuburg have clearly demonstrated that these spores have, besides the features common to the megaspores of all the fossil and recent lycopsids, the few structural characteristics regarded as distinctive of the isoetalean megaspores [43,44]. Besides P. rossica and P. sternbergii, numerous other fossil lycopsids have spores in which papillae and/ or laminated zones-on the basis of examination with LM or TEM, respectively-have been reported. Many of them are regarded as belonging to the Isoetales In Chaloneria cormosa from the Pennsylvanian of North America, the trilete microspores are known to contain three interradial papillae distributed as in Pleuromeia and were compared with the dispersed genus Endosporites [11,45]. There are still many spores reported without possessing papillae. This is ascertained now that such spore structures have never been observed such rare and unique evolutionary strategy in any species of the genus from any part of the world.As mentioned above, a large number of dispersed fossil flora of megaspores have been found resembling the normal megaspores of the extant genus Isoetes [15,40,38,41,42]. Megaspores of Pleuromeia have also been found to correspond with the morphology of Isoetes megaspores. Their ultrastructural similarity appeared to be of great phylogenetical interest because it had suggested close relationships between the Triassic Pleuromeia and the extant Isoetes. Lugardon and associates in their monumental work have discovered several species of Pleuromeia, a very important Triassic genus testifying reduction series of evolution among lycopods, to have produced spores very much similar to the extant genus Isoetes Papers presenting ultrastructural study of the megaspores in Pleuromeia rossica Neuburg have clearly demonstrated that these spores have, besides the features common to the megaspores of all the fossil and recent lycopsids, the few structural characteristics regarded as distinctive of the isoetalean megaspores [43,44]. Besides P. rossica and P. sternbergii, numerous other fossil lycopsids have spores in which papillae and/ or laminated zones-on the basis of examination with LM or TEM, respectively-have been reported. Many of them are regarded as belonging to the Isoetales In Chaloneria cormosa from the Pennsylvanian of North America, the trilete microspores are known to contain three interradial papillae distributed as in Pleuromeia and were compared with the dispersed genus Endosporites [11,45]. There are still many spores reported without possessing papillae. This is ascertained now that such spore structures have never been observed such rare and unique evolutionary strategy in any species of the genus from any part of the world.
Most likely, Isoetes was born in the Permian-Triassic crisis with enormous inherent evolutionary strategies and the genus has had established by Early Triassic by adapting to waterlogged and marshy habitat. The genus Chaloneria described by Pigg and Rothwell (1983) has been reaffirmed as the plausible ancestor of Isoetes, from which the radiation of Triassic lycopsids might have taken place. Retallack (1997) also opined that in the Early Triassic, two distinct adaptive lineage forms have had evolved in the family Isoetaceae; one assuming arborescent habit and the other one growing as short, compact sclerified cone-like fructifications. Isoetes beestonii is the oldest known species of the genus, from which the Triassic lycopods appear to constitute four lineages, viz.: Isoetes, Tomiostrobus, Cyclostrobus and Pleuromeia [11,38,10,45]. Similarly, origin and evolution of heterospory in diverse lineages [42] in barinophytes, lycopsids, sphenopsids, ferns and progymnosperms in different subgroups and at different times during Palaeozoic. The evolution of isoetalean lycopods [37, 46,47,43,44]. which goes back to the Upper Devonian, is represented by Cyclostigma, Clevelandodendron and Lepidosigillaria, in the Carboniferous by Protostigmaria, Paurodendron and Chaloneria, in the Mesozoic by Isoetes/Isoetites, Pleuromeia, Lycostrobus, Nathorstiana and several other genera [11,38,45]. The most significant feature often present in a fossil lycopod megaspore has been the perisporal (perine) flaps or simple “laminate zone”. The proximal face of most megaspores is often covered with either soft mucilaginous perispore (perine) or also has fine tubules or canals. The presence of dentate projections (thick spines) as seen in Isoetes pantii -Chalonospora spores (Table,1: Fig 1 A) or long neck like opening (Fig.2B) and other features however indicate that many fossil genera are being represented in this relic gene combination syndrome. Several studies conducted on fossil spores have never submitted any such observations [32,38,41,31,37,46,47]. There is a future possibility that ongoing research by paleontologists may find a few fossil lycopods from the fossil beds which may depict exact resemblance with these unusual Chalanospores (Figs 1 & 2 in Table 1) Even megaspores of Pleuromeia species may be found to be partly comparable besides with already guessed fossil genera, Nathorstisporites, Nikitinosporites, Paxillitrellites sp., Minerosporites etc.
Megaspores developing inside the heterosporangia have had germinated to produce gametophyte, and a few stages with attached embryo and young sporophytes were also presented along with those spores which were found to germinate within the sporangium and bore archegonium [29,20,16, 20,25]. Though we understand that these unusual mega spores (Fig.1 & 2) and germinated ones are not comparable to any living or fossil lycopod, this is certain that geologically too old “relic” genes have been rejuvenated within the heterosporangia. The presence of twoMegaspores developing inside the heterosporangia have had germinated to produce gametophyte, and a few stages with attached embryo and young sporophytes were also presented along with those spores which were found to germinate within the sporangium and bore archegonium [29,20,16, 20,25]. Though we understand that these unusual mega spores (Fig.1 & 2) and germinated ones are not comparable to any living or fossil lycopod, this is certain that geologically too old “relic” genes have been rejuvenated within the heterosporangia. The presence of two types of unicellular rhizoids evolutionary reminds of genomes of some liverworts like Marchantia and members of Marchantiales) but the rhizoids are reticulate and rough spiny surface is in the inward projection [6,8]. But here, the germinating megaspores bearing young embryo at the top of the spore (Fig. 4 A, B, C) do produce rhizoids from the base of the developing embryo and a few rhizoids have spiny layer on the outer face of the rhizoid (Fig. 4D). Unbelievable though, such rhizoids developing from the embryo which is very typically placed at the upper part of the germinated spore (because, gametophyte develops archegonium at the upper exposed part assume a great evolutionary significance [20,6,8]. We do not have any such observation on record for Isoetes. Additionally, the germinated megaspores show attached embryo (Fig.4A, arrowed) and also exhibit rare category of single cell rhizoid like structures with spiny surface (Fig. 4 B, D, arrow). Such a young exoscopic sporophyte with spiny unicellular rhizoids are unknown except sometimes observed in the related but homosporous lycopod genus Lcopodium (now named, Huperzia) [6,8].
Since pteridophytes are the oldest vascular land plants on the earth, there have been numerous evolutionary strategies in order to attain wider distribution and prolific survival competitions. Extremely rare features [18,28,48]. have been rules rather than exceptions in the plant populations of Isoetes pantii but not in other species Isoetes coromandelina and I. sampathkumaranii which grow intermixed in the same pond at Narsinghgarh in Central India. Numerous genetic mechanisms and ecological and climatic changes have been operative and not only chromosomal changes and natural hybridizations influencing genomic reshuffles [19,49,20], but also, a large number of morphological and physiological transformations have had taken place. Of the most significant among them was origin of heterospory and this is almost certain to believe that atleast there must have been a plant with a sporangium transformed as “heterosporangium” [50,45,42,20]. Selective epigenetic mechanisms should have been operative [27,19,23,51,16,20,25,28]. within this sporangium so as to result in the death of certain spore mother cells, some spore mother cells gradually becoming micro and others as megaspore mother cells. In this process, quite likely, rare genic combinations might have enforced recombinations causing reappearance of those genes which might have been active several millions of years ago. In my opinion, based on consistent and prolonged observations, the reappearance of “heterosporangia” and birth of such fossil like spore within these sporangia is a natural recalled demonstration of origin of heterospory. It is worth reemphasizing that regular and repeated development of highly abnormal spores in different shapes and sizes, but always revealing isoetalean traits over five decades, can never be a nongenetic phenomenon.
While all plants of Isoetes pantii have been observed to inherit the production of polymorphic triradiate tuberculate megaspores inside the megasporangia and polymorphic microspores and large megaspores with thin reticulations inside the heterosporangia, additional and irregular production of these abnormal spores (Fig. 1 & 2) must be due to epigenetic phenomena probably installed due to chromosomal fissions and fusions. Isoetes pantii plants have exhibited 2n=36-39 and 2n=48 chromosomes with new appearance of X, Y and B chromosomes. Plants with these spores (Fig 1& 2) resembling some fossil lycopods (such spores are not produced by any living lycopod) always possess variable 2n=36 to 39 chromosomes [19,14,20]. Strangely, some meiotic preparations even revealed lower counts with many breaks, translocations and small dot chromosomes (Fig 3 A & B). Prevalence of recurrent breaks and variable chromosomes during mitotic and meiotic divisions over these several repeats do confirm that these molecular incidences might have been also responsible for genomic reshuffle.
Since beginning [15,27] this became evident that chromosomal aberrations and variable counts of chromosomes among plants of the same species from the same place of collection do indicate that natural hybridization and or other genetic mechanisms is expressing such variations (evolution of sex chromosomes) which were never reported earlier for any species of the genus. Genomic studies further lead to identification of many DNA sequences which were found to be similar to a few DNA stretches of human genom [22,52] Our studies also had initially suggested that plants do have many sequences akin to those present in human genome. This was explained by an exhaustive survey of sequences in common among many plants, animals and human genome and this was eloquently pointed out that the species Isoetes pantii has evolved with exhibiting unique sequences because of genomic reshuffle. Besides DNA hypomethylation, origin of X and Y chromosome mechanism, we had also suspected genomic imprinting and or any other such molecular mechanism which has rejuvenated “relic” DNA sequences so as to produce heterospory within a sporangium (heterosporangium) and provide different gene combinations to produce so different megaspores (Figs 1 & 2;) [51,25,48]. More sophisticated experimental approaches have to be attempted as there are already definite evidences of X and Y chromosomes possessing unique sequences akin to a part of human chromosome in a liverwort (Marchantia polymorpha) as well as in several other plants [53,54].
Though both microspores as well as megaspores of species of Isoetes developing in the respective sporangia, reported from world over do show partial resemblances and have been compared variously with spores of fossil lycopods but never so far such unusual megaspores, developing inside the heterosporangia not resembling any of the spores produced by any living or fossil genus have been described. These variable unusual megaspores have been named as Chalonospora A few Chalanospores are described which are obviously inherent within the genome. This revitalization of relic genes must be dating back to hundreds of millions of years but we do not know which genera are being represented. However, decidedly Chanospora spp do belong to lycopods (spores being produced inside the Isoetes sporangium are triradiate, measure 150 to 270 mu; have wide perispore as often expected in an aquatic lycopod; dry spores show spines or tubercles or both on proximal or on distal face). This is just thrilling to see that spores which might have been produced hundreds of millions of years ago among giant lycopods and or their other members of declining series, are being produced within the a heterosporangia of a quillwort.
This is well established that habitat destruction, encroachment by anthropogenic activities and more and more dependence on fresh water bodies for agricultural and domestic misuses are destroying a large number of ponds and lakes. All these un-checkable activities along with numerous reproductive and survival problems the plant populations of Isoetes have been declining [17]. Attempts have been made to request a few important villagers to protect certain ponds around Narsinghgarh (parent locality in Madhya, Pradesh, India) so that we can have a look at the wonderful plant, though, gross morphology is just the same as of other species of 16-30 cm size Isoetes plants. Nevertheless isotypes have been deposited in herbaria along with a few slides (Kew; British Museum Nat Hist, London, and other herbaria).
There has been no funding support No liability nor any financial support is declared herewith. There is no potential conflict of interest No need of any consent
I am greatly indebted to my more than a teacher, philosopher and guide, Late Professor W G Chaloner, F. R. S. who had initially encouraged me in 1974 and then our research bonds went on flourishing until his death. He had many times looked at my slides and the material and critically discussed on very relevant topics. Professor Fuchs _Eckert also did the same way and was kind enough to let me examine his enormous collections of the genus Isoetes at his residence at Chur (Trin-vittg) in Switzerland. I am equally grateful to several students and colleagues who helped me in fields and a few of them in laboratories. My thanks are also due to the late Professor Pal at University of Burdwan (Bengal, India) and Professor L Grauvogel-Stamm for help with references.
1. Arnold CA (1947) An introduction to Palaeobotany. New York and London.
2. Seward A C (1933) Plant life through ages. Reprint (1966) by Hafner Publ.Co. New York.
3. Scott D H, Hill T G (1900) The structure of Isoetes hystrix. Ann Bot 14: 413-454.
4. Chaloner W G (1967) Spores and land plant evolution. Rev Pal 1: 89-93.
5. Chaloner W G (1968) The cone of Cyclostigma kiltorkense Haughton from the Upper Devonian of Ireland. Bot J Linn Soc 61: 25-36.
6. Smith G M (1955) Cryptogamic botany. Bryophytes and Pteridophytes. New York and London Vol 2.
7. Chaloner W G (1970) The rise of the first land plants. Biol Review 45: 353-337.
8. Sporne KR (1971) The morphology of pteridophytes. Hutchinson & Co, London.
9. Chaloner W G, Hemsley A R (1991) Heterospory: cul de sac or pathway to the seed. - In: Blackmore S, Barnes, S. H. (eds): Pollen and spores patterns of diversification. Systematics Association Special 44: 151-167.
10. Stewart W, Rothwell GW (1993) Palaeobotany and the evolution of plants. Cambridge Univ Press 2nd Edt.
11. Pigg KB, Rothwell GW (1983) Chaloneria gen nov: Heterosporous lycophytes from the Pennsylvanian of North America. Bot Gaz 144: 32-147.
12. Goswami HK, Arya BS (1968) Heterosporous sporangia in Isoetes. British Fern Gazette (London) 10: 39-40.
13. Goswami HK, Arya B S (1970) A new species of Isoetes from Narsinghgarh, Madhya Pradesh. Journal of Indian Botanical Society 49: 30-37.
14. Goswami HK, Arya BS (2012) Isoetes xpantii (Isoetaceae: Pteridophyta): A Review. Nelumbo 54: 187-192.
15. Goswami H K (1975a) The morphogenetics of mixed sporangia in Isoetes. Journal of Indian Botanical Society 54: 210-218.
16. Goswami HK (2004) Isoetes: IV-reproduction. Bionature, 24:13-24.
17. Goswami HK (2012) Directional selection of Bisexual plants in Natural populations of Isoetes pantii (Pteridophyta: Isoetaceae) with Chromosome Evolution. Acta Botanica Hungarica 54: 103-115.
18. Goswami I, Goswami HK (1986) Genetics of natural variants VI. Some abnormal spores of I. pantii Goswami & Arya resemble fossil lycopods. Bionature 6:23-28.
19. Bhu I, Goswami H K (1990) A new line of chromosomal evolution in Isoetes. Bionature 10: 45-53.
20. Goswami HK, Bhu I (1998). Bisexual sporangia in Isoetes pantii. Bionature 18: 31-36.
21. Goswami H K (2014) Heterosporangium: A New Category of Sporangium in Lycopsida. Acta Botanica Hungarica 56: 77-92.
22. Goswami HK, Chandorkar M S (1994) Highly conserved DNA sequence in Isoetes pantii. Indian Fern Journal 11: 53-55.
23. Goswami HK, Chang SI, Lee IH (2000) Abrupt DNA Methylation might have been responsible for origin of Heterosory. Bionature 20: 1-8.
24. Goswami HK, Bhu I (2000) Isoetes pantii is a Natural hybrid: I. sampathkumarini x I. coromandelina. Bionature 20: 9-18.
25. Bajpai AK, Goswami R, Goswami HK (2004) Isoetes V: Genomic reshuffle: Pertinent DNA sequences from plants and animals. Bionature 1: 25-46.
26. Goswami HK, Mazumdar J (2019) Isoetes Pantii Complex is Evolving with New Basic Chromosome Number. J Adv Plant Sci 2: 1-7.
27. Goswami H K (1975b) Chromosome studies in natural populations of Isoetes pantii with heterosporous sporangia. Cytologia 40: 543-552.
28. Goswami H K (1996) Three decades with Isoetes. Indian Fern Journal 19: 51-61.
29. Goswami HK, Sharma US (1995) Large spores in Microsporangia of Isoetes pantii are megaspores. Indian Fern Journal 12: 195-196.
30. Goswami HK (2011) Population cytogenetic studies confirm X-Y mechanism in Isoetes pantii (Isoetaceae: Pteridophyta). Bionature 31: 13-22.
31. Tryon RM, Lugardon B (1991) Spores of the Pteridophytes. Springer Verlag, NewYork.
32. Srivastava GK, Shukla PK, Wagai SO (1995) Megaspores of some Indian species of Isoetes L and comparable living and fossil forms. Palaeobotanist 44: 215-224.
33. Singh SK , Shukla SK , Dubey NK , Shukla PK (2018) Isoetesxgopalkrishnae (Isoetaceae), a new interspecific sterile hybrid
from central India. Phytotaxa 362: 68-76.
34. Troia A, Pereira JB, Kim C, Taylor, WC (2016) The genus
Isoetes (Isoetaceae): a provisional checklist of the accepted
and unresolved taxa. Phytotaxa 277: 101-145.
35. Troia A, Gabriel J, Taylor WC (2019) A contribution to the
phylogeny and biogeography of the genus Isoetes (Isoetaceae,
Lycopodiidae) in the Mediterranean region. Phytotaxa 395:
168-178.
36. Goswami HK, Patel M (2019) Comparative phylogenetics
of some species of Ophioglossum L. (Ophioglossaceae:
Pteridophyta) in India with comments on evolutionary
significance of high palaeoploidy and rare morphological
traits. The Nucleus 63: 47-58.
37. Taylor WA, Taylor TN (1990) Persistent ultrastructural
features in microspores of heterosporous lycophytes. Grana
29:219-228.
38. Tiwari RM, Awtar R (1987) A palynological assemblage from
Parsora Formation, Johilla Coalfield, South Rewa Gondwana
Basin M P Geophytology 17: 104-109.
39. Goswami HK, Sharma US (1997) Relic Genes in Isoetes
pantii (Goswami & Arya). Bionature 17: 1-6.
40. Banerji J, Jana BN, Maheshwari HK (1984) The fossil flora of Kachchh II. Meszoic megaspores. The Palaeobotanist 33:
190-227.
41. Tiwari R M, Awtar R (1986) Late Permian palynofossils from
the Pali formation, South Rewa Godwana Basin, Madhya
Pradesh. Bull.geol. Min. Matall Sc India 54: 250-255.
42. Kar RK, Dilcher DL (2002) An argument for the origins of
heterospory in aquatic environments. Palaeobotanist 51: 1-11.
43. Lugardon B, Grauvogel-Stamm L, Dobruskina I (1999) The
micro- spores of Pleuromeia rossica Neuburg (Lycopsida;
Triassic]: compara- tive ultrastructure and phylogenetic
implications, CR Acad Sci Paris serie Ila 329: 435-442.
44. Lugardon B, Grauvogel-Stamm L, Dobruskina I (2000)
Comparative ultrastructure of the megaspores of the Triassic
lycopsid Weuromeia rossica Neuburg. Earth and Planetary
Sciences 330: 501-508.
45. Pigg KB (2001) Isoetalean lycopsid evolution: from the
Devonian to the present. Amer Fern J 91: 99-114.
46. Taylor WA (1993) Megaspore wall ultrastructure in Isoetes.
Am J Bot 80: 165-171.
47. Taylor W A (1994) Recognition and characterization of inner
exospore wall layers in modern and fossil lycopsids: the
mesospore. Grana 33: 44-48.
48. Goswami HK, Kang SC, Goswami R, Bajpai AK (2006)
DNA sequences do not abide with phylogenetic distance.
Bionature 26: 1-12.
49. Bhu I, Goswami H K, Sharma US, Bajpai A K (2001) Isoetes
fuchsii: A new Isoetes from India:. Bionature 21: 11-17.
50. Petitt JM (1971) Developmental mechanisms in heterospory
I. Megasporocyte degeneration in Selaginella. Bot J Linn
Soc 64: 237-246.
51. Goswami H K, Lee I H (2001) Is there Genomic imprinting
in Isoetes pantii. Bionature 21: 19-24.
52. Bajpai AK, Goswami H K (2002) Human genome may share
some genes with plant gene pool. Bionature 22: 63-65.
53. Okada SM, Fujisawa T, Sone S, Nakayama R, Nishiyama et
al. (2000) Construction of male and female PAC genomic
libraries suitable for identification of Y chromosome -specific
clones from the liverwort Marchantia polymorpha. Plant J.
24: 421-428.
54. Okada S, T Sone, M Fujisawa, S Nakayama, M Takenaka et
al. (2001) The Y chromosome in the liverwort Marchantia
polymorpha has accumulated unique repeat sequences
harboring a male specific gene. Proc Natl Acad Sci 98: 9454-
9459.
55. Fuchs -Eckert H P (1992) Supplementum and indicem
isoetalium. Bionature 12: 99-159.
56. Kott L S, Britton M S (1982) A comparative study of spore
germination of some Isoetes species of north-eastern North
America. Canad J Botany 60: 1679-1687.
57. Retallack GJ (1997) Earliest Triassic origin of Isoetes and
quillwort evolutionary radiation. J Paleontology 71: 500-521.
