Author(s): Prathima Prabhu Tumkur, Nicole Nazario Bayon, Nithin Krisshna Gunasekaran, Babu R Lamani, Krishnan Prabhakaran, Joseph C Hall and Govindarajan T Ramesh*
In the present study, cellulose nanoparticles were synthesized from waste cotton by acid hydrolysis method. Nanoparticles were obtained by the hydrolysis of pretreated cotton fibers with an acid mixture containing water, hydrochloric acid (HCl) and sulphuric acid (H2SO4). Characterization and biocompatibility studies of obtained cellulose nanoparticles were performed successfully. The morphological studies of cellulose nanoparticles were conducted by Field Emission Scanning Electron Microscopy (FESEM) and Transmission Electron Microscopy (TEM). Both showed that the cellulose nanoparticles synthesized from waste cotton measured approximately 100 - 200nm in diameter. Energy Dispersive X-ray Spectroscopic spectrum showed the elemental composition of the cellulose nanoparticles containing carbon and oxygen. Fourier Transform Infrared (FT-IR) spectroscopy spectrum revealed the presence of several characteristic peaks of cellulose nanoparticles. Human lung epithelial (Beas-2B) cells were used to assess the cytotoxicity and biocompatibility activity of cellulose nanoparticles. Cytotoxicity of cellulose nanoparticles was determined by 3-(4,5- Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay. Live/Dead viability assay was carried out to assess the biocompatibility of cellulose nanoparticles. Generation of Reactive Oxygen Species in Beas-2B cells incubated with cellulose nanoparticles was determined by Reactive Oxygen Species (ROS) assay. MTT and Live/Dead assays showed no significant induction of cell death even at higher concentrations (100 μg) upon exposure to Beas- 2B cells. ROS assay revealed that cellulose nanoparticles did not induce any reactive oxygen species that contribute to the oxidative stress and inflammation leading to various disease conditions. The results revealed that the cellulose nanoparticles have a great potential in a variety of commercial applications.
Natural polymers present in a wide variety of natural organisms carry interesting properties of the respective tissues and similar ones to the extracellular matrix to meet the specific needs of living organisms. These natural polymers, including alginate, chitosan, gelatin, collagen, elastin, starch, and cellulose, have received immense attention by researchers [1]. Cellulose is one among those natural polymers that has grabbed the attention of researchers intensively because of the advantages such as biodegradability, flexibility, low density, low cost and biosafety [2].
Cellulose based nanomaterials such as nanocrystalline cellulose or cellulose nano-whiskers, nano-fibrillated cellulose, and bacterial cellulose possess various potential applications including medicine, energy storage, water treatment, food additives, personal care products, lightweight composites for aerospace and automotive, and building and construction [3-5]. These forms of cellulose impart enormous potential as alternatives to synthetic polymers and offer certain benefits to the composite materials over traditional engineering fibers, e.g., glass and carbon fibers, and mineral fillers such as low density (~1.4 g cc−1) as compared to E-glass (~2.5 g cc−1), comparable specific strength and specific moduli to the glass fibers, and high degree of flexibility [6-8]. Cellulose nanomaterials are taking up an important position in the patent literature than in the peer-reviewed scientific literature as the number of patents filed is increasing each year in a predominant way, illustrating the ongoing race to find success with cellulose based nanomaterials. Until 2015, 475 patents have been 475 patents have been filed that include the words nanocellulose (112 patents), cellulose nanocrystals (38 patents), microfibrillated (water), cellulose (125 patents), bacterial nanocellullose (5 patents), cellulose nanowhiskers (5 patents), and cellulose nanofibers (190 patents). As shown in patent and literature reviews, the use of cellulose nanomaterials in commercial applications need to be explored, even though cellulose nanomaterials hold great potential [5].
Cellulose is the most abundant, renewable and sustainable natural polymer on Earth, with a bioproduction estimated to be over 7.5 × 1010 metric tons annually [9]. Cellulose is present in the plant cell wall along with hemicellulose and lignin where the mechanical properties of natural fibers generally depend on this element. The composition of cellulose fibers consists of microfibrils that, in turn, are made up of elementary fibrils, which are the basic structural units [10,11]. Regardless of its source, cellulose is a fibrous, tough, linear, hydrophilic composed of β-1,4-linked anhydro-D-glucose units and having hydroxyl (-OH) groups that enable cellulose to establish strong hydrogen bonds [12]. Due to the three-alcoholic hydroxyl (–OH) groups, cellulose macromolecules are hydrophilic in nature [9]. Cellulose is a semi-crystalline composite structure having amorphous and crystalline regions in varying proportions depending on the sources. During the hydrolysis process, an unbonded hydroxyl group present in the amorphous region of cellulose plays an important role in their reactive nature whereas the crystalline region of cellulose remains intact, releasing single and well-defined crystals in the form of cellulose nanoparticles [13- 15]. Various extraction processes such as enzymatic hydrolysis, alkaline hydrolysis, mechanical treatments, and electrospinning have been used to produce cellulose nanoparticles, but acid- hydrolysis is the most well-known, efficient, and widely used extraction method. However, it is a pity that direct investigations on the biocompatibility of cellulose nanomaterials are rare. Hence, an extensive characterization and biocompatibility study of nanocellulose that depend largely on the source of cellulose and extraction methods is crucial [11,12].
Synthesis of cellulose nanomaterials has been carried out extensively from a variety of natural sources but the use of cotton for the production of nanocellulose is yet to be widely exploited for the commercial applications. In the present study, nanocellulose was synthesized by acid hydrolysis method from waste cotton. The synthesized nanocellulose was characterized by FESEM, TEM, EDX and FT-IR, and biocompatibility studies were performed using MTT assay, live/dead assay, and ROS assay.
For the synthesis of cellulose nanoparticles by acid hydrolysis method, waste cotton fibers were procured from Indian cotton mills. The chemicals used were a non-ionic surfactant Bioworld wash buffer – Tween 20 (1 g/L), sodium hydroxide (NaOH), hydrochloric acid (HCl) and sulphuric acid (H2SO4) from Fisher Scientific (Newark, NJ, USA). Deionized (DI) water from Millipore Direct-Q UV was used for all the experiments. The Cellulose Ester (CE) dialysis membrane was obtained from Biotech (MWCO 0.5-1.0 kD). Dulbecco’s Modified Eagle Medium (DMEM), Dulbecco’s Phosphate Buffered Saline (DPBS) solution, Fetal Bovine Serum (FBS), penicillin and streptomycin were purchased from Atlanta Biologicals, Inc. (Atlanta, GA, USA). Dimethyl sulfoxide (DMSO), 3-(4, 5-dimethylthiozol- 2-yl)-2, 5-diphenyl tetrazolium bromide (MTT), and Live/Dead viability assay kit were purchased from Sigma Aldrich (St. Louis, MO, USA). All reagents used were of analytical grade.
Cellulose nanoparticles were synthesized as described earlier with certain modifications [16]. Initially, the procured waste cotton fibers free from their seeds were cleaned by removing dirt and any visible wastes. 10 g of cotton fibers were treated with 100 ml of 5 M NaOH solution for 3 h at 78 °C and washed with DI water until the pH reaches neutral. Later, the washed cotton fibers were dried in hot air oven at 90 °C overnight. The dried fibers were then transferred into 100 ml of DMSO for 3 h at 78 °C and washed 5 times in 250 ml of DI water. The resulting cotton fibers were air dried overnight at 90 °C.
Acid hydrolysis was carried out by adding 5 g of pretreated cotton fibers into 150 ml of acid mixture containing DI water: HCl: H2SO4 in the ratio of 6:1:3. This suspension was subjected to hydrolysis at 80 °C for 4 h with constant stirring. At the end of acid hydrolysis, milky colloidal suspension was obtained. The suspension was ultrasonicated for 4 h and then the suspension was transferred to the centrifuge tubes and centrifuged at 5000 rpm for 10 min using Sorvall Legend Mach 1.6R Centrifuge from Thermo Electron Corporation. The obtained hydrolysate was continuously washed with DI water and centrifuged at 5000 rpm for 10 min. The contents in the centrifuge tube were neutralized using 2 N NaOH to pH 7. The neutralized contents were again washed 4 times with 50 ml DI water per wash. Later, the product containing cellulose nanoparticles were dialyzed overnight against DI water using CE dialysis membrane to remove the impurities such as residual salts or acid contents. After dialysis, the nanocellulose suspension was frozen using liquid nitrogen and subjected to freeze drying in Labconco Freeze Dry System for 48 h. The obtained freeze- dried cellulose nanoparticles were further characterized using FESEM, EDX, TEM and FT-IR.
The synthesized cellulose nanoparticles were characterized by FESEM, EDX, TEM and FT-IR.
Prior to FESEM-EDX analysis, the cellulose nanoparticles were dispersed in DI water and then subjected to ultrasonication for 15 mins. After sonication, 50 µl cellulose nanoparticles suspension was added on a carbon tape and air-dried overnight for the analysis. Before examination, a fine layer of gold was sprayed on the samples by an ion sputter coater with a low deposition rate. The morphology and elemental composition of cellulose nanoparticles were determined by FESEM and EDX spectroscopy, respectively. FESEM-EDX analysis was performed at 10 kV.
Imaging was performed using Hitachi TEM. Sample preparation was done by sonicating a solution containing cellulose nanoparticles and DI water. 10 µl of nanoparticle solution was deposited on the surface of the TEM grid made up of copper coated with a thin carbon film. The sample was allowed to dry and then, the structure of the nanoparticle was observed at 100kV.
FT-IR is a great technique to evaluate structural variations on samples due to the chemical treatments. The FT-IR spectrum of the cellulose nanoparticles was obtained using Thermo Nicolet Nexus 670 Fourier Transform Spectrometer in absorbance mode ranging from 4000-500 cm-1 at a resolution of 2 cm-1 with 100 scans per sample. The obtained FT-IR spectrum was analyzed using Thermo Nicolet OMNIC software.
Human lung epithelial cells (Beas-2B) were used in MTT assay, Live/Dead viability assay and ROS assay to perform biocompatibility studies by assessing the cytotoxicity and cell viability in the presence of cellulose nanoparticles.
Beas-2B cells were obtained from American Type Culture Collection (Manassas, VA). The cell lines were revived and cultured as described previously [17]. The cells were seeded on a 75 cm2 cell culture flask in 12 ml DMEM with 10% FBS, 100 IU/mL of penicillin and 100 g/mL of streptomycin and incubated in an incubator with 5% carbondioxide (CO2), 95% humidified atmosphere at 37 °C. The cells were subcultured when they reach a 70–80% confluence to ensure proper growth and health of the cells. For all assays, DPBS was used to prepare the stock of cellulose nanoparticles.
The cytotoxicity of cellulose nanoparticles was determined by using a yellow tetrazolium salt, MTT as described earlier [17]. 100 µl of cell suspension having 5000 cells/ml was seeded in a 96-well plate and incubated in a CO2 incubator maintained at 37 °C, 5% CO2 for 48 hrs. Before adding different concentration of cellulose nanoparticles (100 µl/well), the cells were washed with DPBS. Then, the plate containing cells with nanocellulose was incubated in a 5% CO2 incubator at 37 °C for 48 hours. Later, the plate was again washed with DPBS and the cells in each well of the plate were treated with 25µl MTT (5 mg/ml) and further incubated for 3 hours at 37 °C in a 5% CO2 incubator. After incubation, 100 µl DMSO was added to dissolve the purple color formazan formed inside the live cells of each well and the absorbance was measured at 570nm using Ultraviolet-Visible (UV- VIS) spectrophotometer.
To determine the viability of the Beas-2B cells in the presence of cellulose nanoparticles, Live/Dead viability assay was performed. The live and dead cells were differentiated using the mixture of two dyes namely calcein AM and Ethidium homodimer-1 (EthD-1) in this assay. Calcein AM is a non-fluorescent cell-permeant dye that converts to a green fluorescent molecule by intracellular esterase enzyme in live cells detected at an excitation (ex)/ emission (em) 495 nm/ 515 nm. EthD-1 is a bright red fluorescent dye taken up by membrane damaged dead cells that can be detected at an ex/em 495 nm/ 635 nm. EthD-1 is left out by the intact plasma membrane of live cells.
2ml of Beas-2B cells in the DMEM media (5000 cells/ml) were added to a six well plate and incubated at 37 °C, 5% CO2 for 48 hours in a CO2 incubator. After 48 hours, the cells were gently washed with DPBS and treated with various concentration of cellulose nanoparticles and incubated at 37 °C for 48 hours in a 5% CO2 incubator. The dye mixture was prepared by adding 20 µl of the supplied 2 mM EthD-1 stock solution and 5 µl of the supplied 4 mM calcein AM stock solution to 10 ml of sterile DPBS and vortexed to ensure thorough mixing. 2ml of the resulting solution is then added to the cells and incubated for 30 mins at room temperature. Following the incubation, the stained cells were observed under a fluorescence microscope (Nis Element, Nikon Instruments Inc, Melville) and the image of each well was captured [18].
Briefly, equal numbers (10,000 cells/well) of Beas-2B cells were seeded in a 96-well plate and cultured in a 95% humidified chamber with 5% of CO2 overnight. The cells were then treated with 10µM of DCF-DA (2,7- dichlorofluoroscein diacetate) and incubated in the chamber for 3h. Cells were then washed with phosphate buffered saline (PBS) and treated with varying concentration of cellulose nanoparticles dispersed in DMF. After 48h, the intensity of fluorescence was measured at excitation/ emission wavelength of 485/527 nm respectively and the values represent the fluorescence unit [19].
Cotton contains the highest percentage of cellulose (87-96%) among the various cellulosic sources [20-24]. And it occupies almost 1/3 of the global market of textile fibers with annual output of over 23 million tons per year. Hence waste cotton fiber would be the best source for the large-scale production of cellulose nanoparticles [25].The waste cotton was cleaned by removing the visible dirt. Then, the cleaned cotton fibers were initially made to swell with the help of NaOH and then followed by DMSO treatment. This helps in saccharification of the cotton fibers. The pretreated fibers were hydrolyzed using an acid mixture containing HCl and H2SO4 where a breakage of the characteristic β-1,4- glycosidic bonds takes place. During hydrolysis, the amorphous regions are attacked, leaving the crystalline regions intact. Later, the hydrolyzed products were subjected to ultra-sonication in order to achieve nano-sized particles and then were purified to remove the free acid molecules by dialysis method. This resulted in the synthesis of cellulose nanoparticles.
The current method of synthesis required considerably less time and proved to be cost effective compared to enzymatic method carried out in the previous work [2]. Hence, this method of synthesizing cellulose nanoparticles could be used in commercial applications.
Figure 1 displays FESEM micrograph of cellulose nanoparticles synthesized from cotton, where particles are sputter coated with gold. The nanoparticles are almost spherical in shape with a diameter ranging from 100 – 200nm. Figure 2 shows the TEM micrograph of cellulose nanoparticles where an individual nanoparticle can be observed. Even in TEM micrograph, the morphology of the nanoparticles was nearly spherical with a range of 100-200nm in size which reconfirms the FESEM results. Figure 3 shows the EDX spectrum of cellulose nanoparticles synthesized by acid hydrolysis. The EDX analysis revealed the composition of the elements with absorption peaks representing carbon and oxygen in the spectrum. FT-IR spectroscopy helps to obtain the direct information about changes in chemical functionality which has been extensively used for structural analysis of the nanoparticles. FT-IR spectra of cellulose nanoparticles obtained by acid hydrolysis is shown in Figure 4. The spectral bands at 3153-3486 cm-1 (O-H stretching intramolecular hydrogen bonds for cellulose I), 2883 cm-1 (C-H stretching), 1637 cm-1 (associated with the aromatic ring present in lignin and absorbed water), and 1363 cm−1 (C–H deformation vibration). 1308-1363 cm−1, 1152 cm−1, and 889 cm−1 are the characteristic peaks of the cellulose. Thus, characterization by FESEM, TEM, EDX and FT-IR confirmed the synthesis of cellulose nanoparticles.
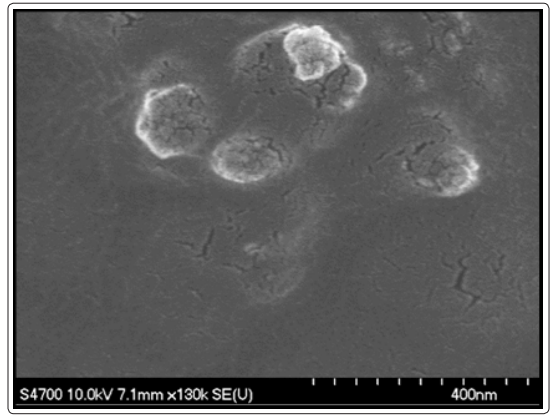
Figure1: FESEM image of synthesized cellulose nanoparticles from cotton fibers
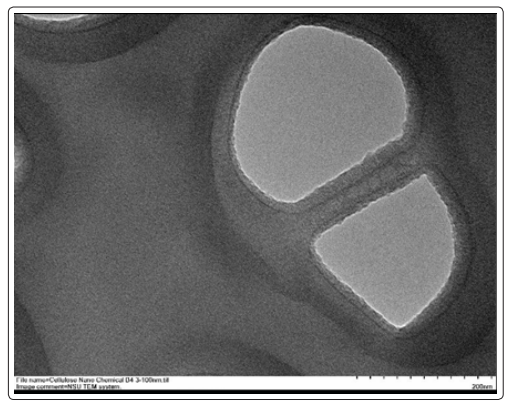
Figure 2: TEM image of synthesized cellulose nanoparticles from cotton fibers
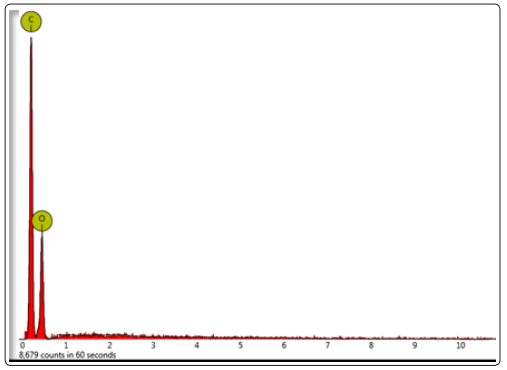
Figure 3: EDX spectrum of synthesized cellulose nanoparticles from cotton fibers
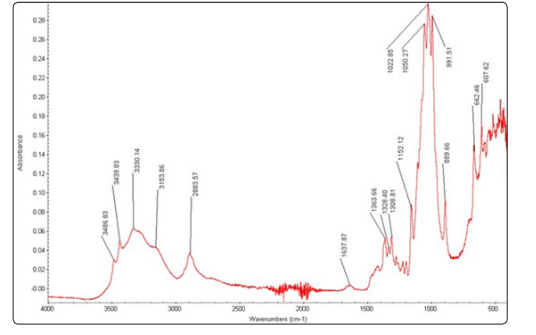
Figure 4: The FT-IR spectrum of chemically synthesized cellulose nanoparticles from cotton fibers
MTT assay was performed to assess the extent of cell damage in the presence of cellulose nanoparticles. The cells were exposed to the varying concentration of the cellulose nanoparticles for 48 hours. Figure 5 shows the MTT assay results in Beas-2B cell line with different concentration of 10, 25, 50, and 100µg/well cellulose nanoparticles respectively. The MTT assay showed that the viability of the cells didn’t decrease in the presence of cellulose nanoparticles. In fact, the cell growth increased as the concentration of the cellulose nanoparticles increased until 50 µg/ well and then became approximately constant. Increase in the cell growth could be because of the larger surface area provided by the nanocellulose aggregates. Thus, implies that the synthesized cellulose nanoparticles are biocompatible. To reconfirm the MTT assay, a live/dead assay was performed using Beas-2B cells. The cells were treated with 5, 10, 25, 50, and 100µg/well of cellulose nanoparticles. The images show the green colored dye taken up by the Beas-2B cells (Figure 6) which clearly indicates no cell death when treated with varying concentration of the cellulose nanoparticles. Live/dead assay results were consistent with the MTT assay.

Figure 5: MTT assay. The MTT dye uptake by Beas-2B cells treated with varying concentration of cellulose nanoparticles was read at 570nm using spectrophotometer. (Absorbance is mean ± 8 wells, 6 experiments performed independently). X-axis: (1) PBS - Negative Control (2) CN10 - 10µg/well (3) CN25 - 25µg/well (4) CN50- 50µg/well and (5) CN100 - 100µg/well, where CN – Cellulose nanoparticles. Y-axis: Cell viability – measurement of optical density at 570nm (absorbance) [17].
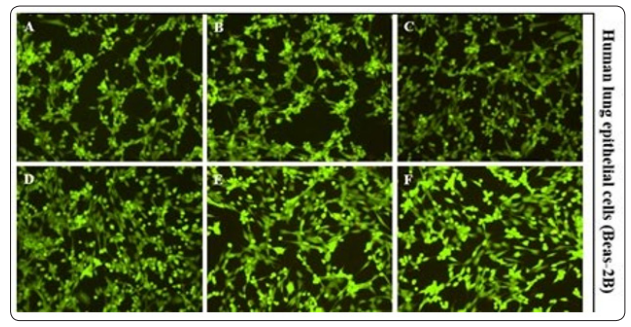
Figure 6: Live/Dead assay. Images of stained Beas-2B cells in the presence of varying concentration of cellulose nanoparticles (Performed 6 times individually). (A) PBS - Negative control (B) CN - 5µg/well (C) CN - 10µg/well (D) CN - 25µg/well (E) CN - 50µg/ml and (F) CN - 100µg/well where CN – cellulose nanoparticles [18].
Oxygen radicals collectively called as reactive oxygen species play a key role in cytotoxicity. Increased ROS levels in cells by a chemical compound reflects toxicity and cell death. The intensity of fluorescence in ROS assay was measured at excitation and emission of wavelength at 485/527 nm, respectively and expressed as fluorescence units. With the addition of the cellulose nanoparticles, the amount of ROS produced by the cells was relatively stable. Hence, the cell growth didn’t decrease in the presence of cellulose nanoparticles and was approximately same as the negative control (PBS). The intensity of fluorescence was measured at excitation and emission of wavelength at 485/527 nm, respectively and expressed as fluorescence units. ROS assay result was also consistent with MTT assay showing no cell death with varying concentration of cellulose nanoparticles (Figure 7).
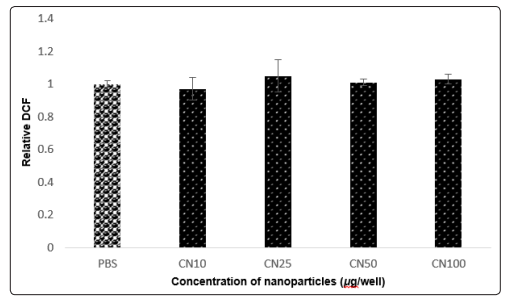
Figure 7: ROS assay. The intensity of fluorescence by the Beas- 2B cells incubated with cellulose nanoparticles was measured at excitation and emission of wavelength at 485/527 nm, respectively and expressed as fluorescence units. X-axis: (1) PBS - Negative Control (2) CN10 - 10µg/well (3) CN25 - 25µg/well (4) CN50- 50µg/ well and (5) CN100 - 50µg/well where CN – cellulose nanoparticles. Y-axis: Relative DCF represents the presence of live cells [19].
Cellulose nanoparticles were synthesized successfully by acid hydrolysis. FESEM and TEM analysis revealed that the range of an average size of the nanoparticle is around 100 – 200nm. EDX and FT-IR analysis showed that the synthesized cellulose nanoparticles were pure in form. Biocompatibility studies carried out by MTT, ROS and live/dead assays revealed that the cellulose nanoparticles were compatible with human lung epithelial cells. Compared to enzymatic process, chemical-mediated process produced high yield at low cost and time. In the future work, the synthesized cellulose nanoparticles will be further utilized to explore the biomedical and energy storage applications.
This research work was supported by NSF-CREST RD-1547771 grant. We also thank Mrs. Olga Trofimova at William and Mary, Williamsburg, VA for FESEM, EDX and FT-IR experiments. We also thank Dr. Greene, Lesley H. at Old Dominion University, Norfolk, VA for her help in freeze drying process.
