Author(s): Ioanna Ioannou* and Artemis Giotsa
Background: The hospitalization of preterm infants in Neonatal Intensive Care Unit (NICU) is generally an unexpected event that can be stressful experience for families and especially for parents.
Objective: To assess the anxiety levels in parents with preterm infants hospitalized in NICU and determine its association with clinical and sociodemographic variables.
Method: This prospective, follow up–cohort study was conducted at the NICU of a tertiary maternity hospital in Athens, Greece during 18/12/2019 to 31/12/2022. A total of 120 parents, whose infants had birth weight (BW)<1750 grams (g) and gestational age (GA)<34 weeks (w) were involved. The data collected by a demographic form and the State-Trait Anxiety Inventory Scale (STAI).
Results: Our data showed that the STAI score for state anxiety to mothers was significantly higher in the 1st assessment (Mean±SD:52.2±11.9) than 2nd (Mean±SD:47.8±12) and 3rd (Mean±SD:49±10.3). The STAI score for trait anxiety was similar, in particular it was significantly higher in the 1st assessment (Mean±SD:46.6±14.6) compared to 2nd (Mean±SD:41.9±14) and 3rd (Mean±SD:42.8±13). Also, the STAI score for state anxiety to fathers was significantly higher during the 1st assessment (Mean±SD:47.1±12.2) compared to the other two assessments (Mean±SD:42.8±9.8 & Mean±SD:41.7±9.1 respectively), while trait anxiety didn’t show significant variability.
Conclusion: Our findings suggested that the premature birth and infant’s hospitalization might have a negative effect on parents’ emotional state. Identifying which mothers and fathers are at risk immediately after birth and during NICU stay could help to direct specific interventions that can prevent them of stressful feelings.
Preterm birth is an important issue in public health and a major part of worldwide neonatal mortality and morbidity. According to World Health Organisation (2018), preterm infants are born before 37 weeks of gestation (<259 days). Due to advanced and improved perinatal care, the incidence of preterm birth is increasing worldwide, while perinatal mortality decreasing. It is estimated that fifteen million preterm infants are born every year, and an estimated 50% or more require care in a NICU, specialised care by specialists and equipment for care of preterm infants. Preterm birth is a multi-problematic event that may cause different consequences for infants’ and parents’ experiences. It is widely demonstrated that preterm birth is associated with several poor infants’ neurodevelopmental outcomes, and particularly in infants with BW<1500g and GA<32w [1-8].
Parents of preterm infants who admitted at NICU experience increased anxiety and stress levels due to a range of factors including the NICU environment, feelings of powerless and disruptions in the parental role [9,10]. In fact, premature birth requires early separation from its parents and long-term admission to NICU. Additionally, the possible critical condition both of the infant and mother may sock parents, turning preterm birth in a stressful or traumatic event for both of them [11,12]. Premature infants’ admission in the NICU is a psychological crisis for parents-family, they are confronted with neonatal complications, possibly causing long-term effects and fear of losing their baby. Feelings of guilty and uncertainty about their infant’s prognosis may continue after discharge. As a consequence, preterm parents are at greater risk for mental health, such as anxiety, stress and depression [13-16].
According to older record, the major sources of anxiety in parents of premature infants during the first month of their life are:
i) personal and family factors,
ii) prenatal and postnatal experiences,
iii) the illness,
iv) concern about the infants’ health improvement,
v) loss of parental role and vi) the health care provided. In view of these emotional circumstances, which may have short-term and long–term impact, it is necessary to develop effective interventions to offer targeted support to parents of the infants admitted in the NICU [17,18,19]. It’s important all health professionals who attending to an infant also deliver comprehensive care to the parents [20]. Health professionals need to be trained to examine the backgrounds, personal circumstances, concerns and fears of every family hospitalized in NICU, in order to intervene effectively when it required.
The main objective of this study was to determine the anxiety levels of parents immediately after the premature birth of their infants admitted to the NICU and identify associated factors.
This study is a prospective, follow up-cohort study conducted over three-year period (18/12/2019 to 31/12/2022). The participants in this study were parents whose infants were admitted to the NICU of a tertiary maternity hospital in Athens, Greece. Parents participated in the study had at least one infant who immediately admitted to the NICU as a result of prematurity, related disease and other severe complications. During the study period, 65 couples of parents were recruited, three of whom had preterm infants who needed to be transferred to other NICUs and two had lost their infants. The final sample was 60 couples, whose infants had BW<1750g and GA<34w and with anticipated stay in NICU of greater than 45 days, in order to have enough time to complete the project. Also, inclusion criteria for this study were: i) Parent’s age at least eighteen years old, ii) Parent’s good knowledge of Greek language and iii) Absence of parent’s mental illness.
This study was approved by the Ethical Committee of University of Ioannina in December 2021 and related permission was obtained from the Maternity Hospital, in December 2019, where the study took place. Also, all participants were informed about the purpose of the study, assured about their anonymity and informed that they wouldn’t be commitment and could withdraw from the study whenever they wished.
To assess parental anxiety was used the State-Trait Inventory Scale by C.D. Spielberger-STAI (1983). STAI is well-established instrument and reliable to measure anxiety in adults. This inventory manages the distinguish between state anxiety and, personality trait anxiety. For this purpose, has two subscales, the first measures state anxiety (STAI-X-1) and second measures trait anxiety (STAI-X-2). The STAI is a 40-item self-report scale with four response options for each of them. The questions are equally divided into positive and negative responses of emotion. Responses are scored on a Likert-type four-point rating scale, which for state anxiety measures intensity (not at all, somewhat, moderately so and very much so) and trait anxiety measures frequency (almost never, sometimes, often and almost always). With the maximum score of 80, higher scores indicate greater symptoms of anxiety. The parents who scored in each sub-scale >40 was considered to manifest symptoms of state or trait anxiety. In particular, a score less than forty is considered normal (no anxiety), forty to forty-seven is considered mild anxiety, forty-eight to fifty-five as moderate anxiety and a score under fifty-five as severe anxiety [21]. This scale has been translated and adapted into Greek by Liakos A. & Giannitsi S. in 1984 but the validity and reliability check was later carried out by Anagnostopoulou T & Kioseoglou G in 2002. The State and Trait subscales show excellent internal consistency with Cronbach’s a coefficient of 0.938 and 0.905, respectively [22,23].
There are many early warning scales that have been implemented to address the global burden of neonatal mortality in NICUs. One of them is the Apgar score that proposed in 1952 by Virginia Apgar as a convenient method for reporting the clinical status of newborn infants immediately after birth and the response to resuscitation if needed. Apgar score is an accepted pattern of assessment and authorised by the American College of Obstetricians and Gynaecologists and American Academy of Pediatrics [24,25]. This score estimated using five parameters, such as regularity and strength of heart rate, breathing effort or maturity of lungs, muscular tone and movement, respiratory or skin colour and reflexes as a response to stimulation. Each parameter is scored zero, one & two. The Apgar score ranges from zero to ten. A total score over seven is considered as “normal” while a total score under seven is considered as “low” that may indicate depressed vitality. The score is recorded twice at first and fifth minute in all newborns with expanded recording at 10-minute for them who scored as seven or less at fifth minute, and in those requiring resuscitation as a method for monitoring response [26,27,28]. Apgar score was developed primarily to assess term infants when the neonatal mortality was very high among preterm infants [29,30].
On that account, scientists used the Clinical Risk Index for Babies (CRIB) score. CRIB score is a scoring system widely used in NICUs. It was published in 1993 by International Neonatal Network after its initial validation [31]. In this study used second edition that identifying the initial mortality risk and illness severity within one hour of admission. This edition contains only five parameters: birth weight, gender, gestational age, body temperature on admission and base excess. The total CRIB II score ranged from 0 to 27. The score has been classified into four levels (Level 1: 0-5, Level 2: 6-10, Level 3: 11-15 & Level 4: above 15). The lower score identifies the better prognosis, and higher score indicates the worst prognosis. It is only applicable to preterm neonates with BW<1500g, as well as those with the GA<31 weeks. CRIB II score is an important indicator in predicting the severity of disease and even infant’s death [32-34].
A standard survey form was constructed by the researchers to record demographic data and social factors. Also, a perinatal form for parents was constructed to collect data on participants’ health status and habits and another perinatal form was constructed to collect data for infants’ health status and clinical risk.
Parents who met the inclusion criteria singed a written consent form before their participation in the study. The demographic data were collected before the administration of the screening scales. Participants completed STAI self-report in the Maternity Ward in 3-4 days after infants’ admission to NICU, 20th-25th day postpartum and before infant’s discharged of the hospital. The follow-up was conducted by self-report screening at their home.
Variables were first tested for normality using the Kolmogorov- Smirnov criterion. Quantitative variables were expressed as mean (Standard Deviation) or as median (interquartile range). Qualitative variables were expressed as absolute and relative frequencies. Repeated measurements analysis of variance (ANOVA) was adopted to evaluate the changes observed in anxiety scores over the follow up period, between parents and across sample’s characteristics. In order to find independent factors that were associated with anxiety scales at each assessment, linear regression analysis models were conducted with a stepwise method from which regression coefficients (b) and their standard errors (SE) were emerged. All reported p values are two-tailed. Statistical significance was set at p<0.05 and analyses were conducted using SPSS statistical software (version 26.0).
Data from 60 labors were recorded. Sample’s characteristics are presented in table 1. Mean mother’s age was 33.6 years (SD=6.7 years), and mean father’s age was 37.2 years (SD=5.6 years). Primiparous were 73.3% of the women, while in 85% of the cases conception was spontaneous. In 90% of the cases, they gave birth with a caesarian section and 76.7% had a single pregnancy.
|
|
N (%) |
|
Mothers’ characteristics |
|
|
Mothers’ age. mean(SD) |
33.6 (6.7) |
|
Educational level |
|
|
Primary |
0 (0.0) |
|
Secondary |
13 (21.7) |
|
University |
45 (75.0) |
|
MSc |
2 (3.3) |
|
Family income |
|
|
<12.000 euro |
14 (23.3) |
|
12.000 - 23.999 euro |
33 (55.0) |
|
24.000 - 35.999 euro |
12 (20.0) |
|
35.000 - 47.999 euro |
1 (1.7) |
|
Family status |
|
|
Married |
51 (85.0) |
|
Single |
3 (5.0) |
|
Cohabitation |
6 (10.0) |
|
Health problem |
17 (28.3) |
|
Smoking |
|
|
Νο |
48 (80) |
|
Yes |
11 (18.3) |
|
Vaping |
1 (1.7) |
|
Fathers’ characteristics |
|
|
Age (years). mean(SD) |
37.2 (5.6) |
|
Educational level |
|
|
Primary |
2 (3.3) |
|
Secondary |
23 (38.3) |
|
University |
35 (58.3) |
|
Pregnancy and labol characteristics |
|
|
Primiparous |
44 (73.3) |
|
Conception |
|
|
Spontaneous |
51 (85.0) |
|
IVF |
9 (15.0) |
|
Labor |
|
|
Vaginal |
6 (10.0) |
|
Caesarian section |
54 (90.0) |
|
Pregnancy |
|
|
Single |
46 (76.7) |
|
Twin |
12 (20.0) |
|
Multiple |
2 (3.3) |
Characteristics of newborns are presented in table 2. Mean gestational age was 29.5 weeks (SD=2.3w) and mean birth weight was 1230g (SD=335g). Mean hospitalization duration was 63.8 days (SD=27.7 days).
|
|
Mean (SD) |
Median (IQR) |
|
Gestational age at labor (weeks) |
29.5 (2.3) |
29.7 (28.3-31.1) |
|
Head circumference (cm) |
26.8 (2.5) |
27 (25-28.3) |
|
Birth weight (gr) |
1230 (335) |
1280 (990-1459.5) |
|
Height (cm) |
35.6 (5.7) |
36 (32-40) |
|
CRIP II SCORE |
6.9 (3.7) |
6.5 (3-9.5) |
|
Predicted death rate |
8.7 (14.3) |
3.5 (0.6-10.1) |
|
Apgar Score (1min) |
6.2 (1.6) |
7 (5-7) |
|
Apgar Score (5min) |
7.8 (1.2) |
8 (7-9) |
|
Hospitalization duration (days) |
63.8 (27.7) |
60 (44-80) |
Mothers’ trait score in total sample and by under study characteristics is presented in table 3. In all mothers, the trait anxiety score decreased significantly from 1st week (3-4 days) to 1st month (20th-25th day) and overall, from 1st week to discharge, indicating a significant reduction in symptoms of trait anxiety (Mean±SD:46.6±14.6, Mean±SD:41.9±14, Mean±SD:42.8±13, respectively). At 3rd assessment, trait score was found to differ significantly only by the number of pregnancies, with primiparous women having significantly more trait anxiety symptoms compared with women who weren’t primiparous (p=0.041). With the other data being available in the table 3, such as mother’s age, type of conception, infant’s birth weight, duration of hospitalization, CRIB II score, Apgar score at 1? & 5? and mother’s health problems, no significant differences were found in the scores of trait anxiety in any of the assessments. The trait score at 1st month and discharge was significantly lower compared to 1st week, regardless of infant’s birth weight, CRIB II score, Apgar score at 1? & 5? and the presence or not of a health problem in mothers. Also, mother’s trait score was significantly decreased at 1st month and discharge compared with 1st week in women who were at least 33 years old, in single pregnancies, in primiparous women, in cases with spontaneous conception and in cases with duration of hospitalization at least 62 days. The trait score was significantly decreased from 1st week to discharge in women with IVF conception. The degree of reduction in score of trait anxiety didn’t differ significantly from the variables in the table 3.
|
|
|
Mothers’trait score |
P2 |
P3 |
||||
|
|
|
1st week |
1st month |
At discharge |
|
|
|
|
|
|
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
1stweek vs 1st month |
1st week vs at discharge |
1st month vs at discharge |
|
|
Total sample |
|
46.6 (14.6) |
41.9 (14) |
42.8 (13) |
0.001 |
<0.001 |
0.871 |
- |
|
Mothers’ age |
<33 years |
47.7 (13) |
44.1 (12.5) |
45.1 (11.1) |
0.077 |
0.070 |
>0.999 |
0.411 |
|
≥33 years |
45.3 (16.4) |
39.7 (15.4) |
40.3 (14.5) |
0.004 |
<0.001 |
>0.999 |
||
|
P1 |
0.519 |
0.226 |
0.162 |
|
|
|
||
|
Primiparous |
No |
50.1 (14.7) |
46.8 (14.6) |
48.4 (12.7) |
0.433 |
0.881 |
0.897 |
0.387 |
|
Yes |
45.3 (14.6) |
40.2 (13.5) |
40.7 (12.6) |
0.001 |
<0.001 |
>0.999 |
||
|
P1 |
0.259 |
0.104 |
0.041 |
|
|
|
||
|
Single pregnancy |
No |
47.1 (16.7) |
42.1 (14.9) |
43.1 (14.1) |
0.125 |
0.074 |
>0.999 |
0.953 |
|
Yes |
46.4 (14.2) |
41.9 (13.9) |
42.7 (12.8) |
0.004 |
0.001 |
>0.999 |
||
|
P1 |
0.881 |
0.967 |
0.926 |
|
|
|
||
|
Conception |
Spontaneous |
47.2 (14) |
42.6 (13.9) |
43.9 (12.8) |
0.002 |
0.001 |
0.450 |
0.461 |
|
IVF |
42.8 (18.2) |
38.1 (15.1) |
36.7 (13) |
0.375 |
0.018 |
>0.999 |
||
|
P1 |
0.407 |
0.379 |
0.127 |
|
|
|
||
|
Hospitalization duration |
<62 days |
45.2 (14.6) |
41.8 (14.6) |
42.8 (13.4) |
0.143 |
0.154 |
>0.999 |
0.273 |
|
P1 |
0.486 |
0.956 |
0.980 |
|
|
|
||
|
Birth weight |
<1235 gr |
46.1 (14.2) |
41.5 (12.8) |
41.8 (12.4) |
0.021 |
0.002 |
>0.999 |
0.775 |
|
≥1235 gr |
47 (15.3) |
42.4 (15.4) |
43.7 (13.7) |
0.020 |
0.023 |
>0.697 |
||
|
P1 |
0.814 |
0.813 |
0.576 |
|
|
|
||
|
CRIB II SCORE |
<7 |
45.1 (15) |
40.8 (14.4) |
41.7 (13.1) |
0.048 |
0.027 |
>0.999 |
0.884 |
|
≥7 |
47.8 (14.5) |
42.9 (13.9) |
43.7 (13) |
0.008 |
0.002 |
>0.999 |
||
|
P1 |
0.485 |
0.568 |
0.565 |
|
|
|
||
|
Apgar score 1’ |
<7 |
45.9 (13.6) |
40.7 (12.7) |
41.6 (11.5) |
0.010 |
0.003 |
>0.999 |
0.786 |
|
≥7 |
47.2 (15.7) |
43 (15.2) |
43.8 (14.3) |
0.035 |
0.015 |
>0.999 |
||
|
P1 |
0.735 |
0.521 |
0.517 |
|
|
|
||
|
Apgar score 5' |
<8 |
49.5 (14.7) |
43.2 (14) |
45.1 (12) |
0.006 |
0.009 |
0.488 |
0.403 |
|
≥8 |
44.9 (14.6) |
41.2 (14.1) |
41.5 (13.5) |
0.035 |
0.005 |
>0.999 |
||
|
P1 |
0.252 |
0.601 |
0.306 |
|
|
|
||
|
Mother’s health problem |
No |
46.7 (15.1) |
42.6 (13.8) |
43.4 (13.3) |
0.011 |
0.004 |
>0.999 |
0.614 |
|
Yes |
46.2 (13.9) |
40.4 (14.8) |
41.2 (12.5) |
0.029 |
0.008 |
>0.999 |
||
|
P1 |
0.902 |
0.587 |
0.556 |
|
|
|
||
1p-value for group effect 2 p-value for time effect 3p-value from repeated measures ANOVA, regarding time*group effect
Fathers’ trait anxiety score in total sample and by under study characteristics is presented in table 4. In all fathers, the trait anxiety score presented a similar score during the follow-up. Trait score at 1st month was found to differ significantly only by maternal age, in cases where mother’s age was at least 33 years old, fathers had significantly more trait anxiety symptoms (p=0.032). With the other variables being available in the table 4, such as number of infants, single pregnancy or not, type of conception, infant’s birth weight, duration of hospitalization, CRIB II score, Apgar score at 1? & 5? and mother’s health problems, no significant differences were found in the scores of trait anxiety in any of the assessments. Also, the degree of change in scores of trait anxiety differed significantly only according from the maternal age (p=0.007). Specifically, when mothers were at least 33 years old, fathers’ trait anxiety score increased, whereas mothers were over 33 years old, fathers’ trait anxiety scores decreased. The degree of reduction in score of trait anxiety didn’t differ significantly from the variables in the table 4.
|
|
|
Fathers’ trait anxiety |
P2 |
P3 |
||||
|
|
|
1st week |
1st month |
At discharge |
|
|
|
|
|
|
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
1stweek vs 1st month |
1St week vs at discharge |
1st month vs at discharge |
|
|
Total sample |
|
36.9 (10.4) |
36.6 (9.7) |
36.5 (10.8) |
>0.999 |
>0.999 |
>0.999 |
- |
|
|
<33 years |
37 (9.7) |
39.1 (10.7) |
38.9 (11.6) |
0.344 |
0.443 |
>0.999 |
0.007 |
|
Mothers’ age |
≥33 years |
36.8 (11.3) |
33.8 (7.7) |
33.9 (9.5) |
0.086 |
0.096 |
>0.999 |
|
|
P1 |
0.940 |
0.032 |
0.076 |
|
|
|
|
|
|
Primiparous |
No |
38.8 (11.1) |
40.4 (12) |
40.4 (13.5) |
>0.999 |
>0.999 |
>0.999 |
0.256 |
|
Yes |
36.3 (10.2) |
35.2 (8.4) |
35.1 (9.5) |
>0.999 |
0.881 |
>0.999 |
||
|
P1 |
0.419 |
0.065 |
0.095 |
|
|
|
|
|
|
Single pregnancy |
No |
35.8 (10.6) |
35.8 (9.5) |
36.8 (11.4) |
>0.999 |
>0.999 |
>0.999 |
0.579 |
|
Yes |
37.3 (10.4) |
36.8 (9.8) |
36.4 (10.8) |
>0.999 |
>0.999 |
>0.999 |
||
|
P1 |
0.641 |
0.739 |
0.911 |
|
|
|
|
|
|
Conception |
Spontaneous |
36.9 (10.2) |
37 (10.1) |
37.1 (11) |
>0.999 |
>0.999 |
>0.999 |
0.155 |
|
IVF |
37.2 (11.9) |
34 (7.1) |
32.9 (9.8) |
0.634 |
0.242 |
>0.999 |
||
|
P1 |
0.929 |
0.396 |
0.282 |
|
|
|
|
|
|
Hospitalization duration |
<62 days |
36.2 (10) |
36.3 (9.2) |
35.6 (10.7) |
>0.999 |
>0.999 |
>0.999 |
0.671 |
|
≥62 days |
37.6 (10.9) |
36.7 (10.3) |
37.3 (11.1) |
>0.999 |
>0.999 |
>0.999 |
||
|
P1 |
0.622 |
0.875 |
0.548 |
|
|
|
|
|
|
Birth weight |
<1235 gr |
37.6 (10.1) |
38.1 (9.8) |
38.2 (10.6) |
>0.999 |
>0.999 |
>0.999 |
0.403 |
|
≥1235 gr |
36.3 (10.8) |
35 (9.5) |
34.8 (11) |
>0.999 |
0.852 |
>0.999 |
||
|
P1 |
0.624 |
0.218 |
0.228 |
|
|
|
|
|
|
CRIB II SCORE |
<7 |
36 (10.9) |
34.8 (9.6) |
34.4 (11) |
>0.999 |
0.844 |
>0.999 |
0.437 |
|
≥7 |
37.7 (10.1) |
38 (9.7) |
38.2 (10.6) |
>0.999 |
>0.999 |
>0.999 |
||
|
P1 |
0.534 |
0.202 |
0.186 |
|
|
|
|
|
|
Apgar score 1’ |
<7 |
38.3 (10.9) |
37.7 (9.7) |
38.6 (9.8) |
>0.999 |
>0.999 |
0.798 |
0.507 |
|
≥7 |
35.8 (9.9) |
35.6 (9.8) |
34.7 (11.6) |
>0.999 |
>0.999 |
0.732 |
||
|
P1 |
0.363 |
0.403 |
0.168 |
|
|
|
|
|
|
Apgar score 5’ |
<8 |
39.5 (11.9) |
38.2 (10.4) |
39.9 (10.7) |
>0.999 |
>0.999 |
0.204 |
0.326 |
|
≥8 |
35.6 (9.4) |
35.7 (9.3) |
34.7 (10.6) |
>0.999 |
>0.999 |
0.431 |
||
|
P1 |
0.167 |
0.340 |
0.078 |
|
|
|
|
|
|
Mother’s health problem |
No |
37.4 (10.6) |
37.2 (10.4) |
37.1 (11.5) |
>0.999 |
>0.999 |
>0.999 |
0.846 |
|
Yes |
35.8 (10.1) |
34.9 (7.6) |
34.9 (9.2) |
>0.999 |
>0.999 |
>0.999 |
||
|
P1 |
0.607 |
0.406 |
0.472 |
|
|
|
|
|
1p-value for group effect 2 p-value for time effect 3p-value from repeated measures ANOVA, regarding time*group effect
Mothers’ state anxiety score in total sample and by under study characteristics is presented in table 5. In all mothers, the state anxiety score decreased significantly from 1st week to 1st month and overall, from 1st week to discharge, indicating a significant reduction in symptoms of state anxiety (Mean±SD:52.2±11.9, Mean±SD:47.8±12, Mean±SD:49±10.3, respectively). With the other variables being available in the table 5, no significant differences were found in the score of state anxiety in any of the assessments. At 1st month state score was found to decrease significantly compared with 1st week, in older mother (mother’s age≥33), in primiparous women, in single pregnancies, in those who conceived spontaneous, in those cases that duration of hospitalization was at least 62 days, infant’s CRIB II score≥7 and infant’s Apgar score at 5?<8 (p=0.020; p=0.015; p=0.045; p=0.013; p=0.036; p=0.026; p=0.047, respectively). Scores of maternal state anxiety were significantly decreased from 1st week to discharge in primiparous women, in those cases with birth weight<1235g and CRIB II score≥7, the state score decreased significantly from 1st week to discharge (p=0.029; p=0.024; p=0.030, respectively). The degree of reduction in symptoms of state anxiety didn’t differ significantly with the variables in the table 5.
|
|
|
Mothers’state anxiety |
P2 |
P3 |
||||
|
|
|
1st week |
1st month |
At discharge |
|
|
|
|
|
|
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
1stweek vs 1st month |
1St week vs at discharge |
1st month vs at discharge |
|
|
Total sample |
|
52.2 (11.9) |
47.8 (12) |
49 (10.3) |
0.013 |
0.039 |
0.862 |
- |
|
|
<33 years |
52.8 (11.3) |
49.8 (10.8) |
50.2 (9.8) |
0.497 |
0.424 |
>0.999 |
0.478 |
|
Mothers’ age |
≥33 years |
51.7 (12.7) |
45.6 (12.9) |
47.8 (10.9) |
0.020 |
0.117 |
0.562 |
|
|
P1 |
0.719 |
0.171 |
0.387 |
|
|
|
|
|
|
Primiparous |
No |
51.9 (14.9) |
49.3 (14.1) |
50.6 (9.8) |
>0.999 |
>0.999 |
>0.999 |
0.600 |
|
Yes |
52.4 (10.8) |
47.2 (11.2) |
48.5 (10.6) |
0.015 |
0.029 |
>0.999 |
||
|
P1 |
0.889 |
0.555 |
0.477 |
|
|
|
|
|
|
Single pregnancy |
No |
52.4 (14.5) |
47.6 (11.8) |
47.1 (11.8) |
0.398 |
0.147 |
>0.999 |
0.647 |
|
Yes |
52.2 (11.2) |
47.8 (12.2) |
49.6 (9.9) |
0.045 |
0.228 |
0.576 |
||
|
P1 |
0.965 |
0.940 |
0.439 |
|
|
|
|
|
|
Conception |
Spontaneous |
53.3 (10.9) |
48.5 (12) |
50 (9.9) |
0.013 |
0.060 |
0.647 |
0.731 |
|
IVF |
46.1 (15.8) |
44 (11.7) |
43.3 (11.6) |
>0.999 |
>0.999 |
>0.999 |
||
|
P1 |
0.094 |
0.308 |
0.072 |
|
|
|
|
|
|
Hospitalization duration |
<62 days |
50.9 (11.7) |
47.5 (11.8) |
48.8 (9.4) |
0.376 |
0.768 |
>0.999 |
0.649 |
|
≥62 days |
53.5 (12.1) |
48 (12.3) |
49.2 (11.3) |
0.036 |
0.053 |
>0.999 |
||
|
P1 |
0.404 |
0.869 |
0.883 |
|
|
|
|
|
|
Birth weight |
<1235 gr |
52.2 (13) |
48.3 (11.2) |
47.4 (11.5) |
0.215 |
0.024 |
>0.999 |
0.235 |
|
≥1235 Gr |
52.3 (10.9) |
47.3 (12.9) |
50.7 (8.9) |
0.072 |
>0.999 |
0.118 |
||
|
P1 |
0.983 |
0.757 |
0.215 |
|
|
|
|
|
|
CRIB II SCORE |
<7 |
49.6 (12) |
46.4 (12.5) |
47.9 (9.9) |
0.501 |
>0.999 |
>0.999 |
0.503 |
|
≥7 |
54.4 (11.5) |
48.9 (11.6) |
49.9 (10.8) |
0.026 |
0.030 |
>0.999 |
||
|
P1 |
0.115 |
0.425 |
0.458 |
|
|
|
|
|
|
Apgar score 1’ |
<7 |
52.6 (12.4) |
47.2 (11.3) |
48.5 (10.2) |
0.054 |
0.093 |
>0.999 |
0.759 |
|
≥7 |
51.9 (11.6) |
48.3 (12.7) |
49.5 (10.6) |
0.256 |
0.486 |
>0.999 |
||
|
P1 |
0.822 |
0.734 |
0.730 |
|
|
|
|
|
|
Apgar score 5’ |
<8 |
54.4 (12.4) |
48 (12) |
51.9 (9.9) |
0.047 |
0.723 |
0.163 |
0.332 |
|
≥8 |
51.1 (11.6) |
47.6 (12.1) |
47.5 (10.3) |
0.213 |
0.079 |
>0.999 |
||
|
P1 |
0,308 |
0,901 |
0,121 |
|
|
|
|
|
|
Mother’s health problem |
No |
52.6 (12.8) |
49 (11.8) |
49.1 (10.8) |
0.142 |
0.073 |
>0.999 |
0.381 |
|
yes |
51.4 (9.6) |
44.8 (12.2) |
48.8 (9.3) |
0.070 |
0.837 |
0.209 |
||
|
P1 |
0.740 |
0.232 |
0.922 |
|
|
|
|
|
1p-value for group effect 2 p-value for time effect 3p-value from repeated measures ANOVA, regarding time*group effect
Fathers’ state anxiety score in total sample and by under study characteristics is presented in table 6. In all fathers, the state anxiety score decreased significantly from 1st week to 1st month and overall, from 1st week to discharge, indicating a significant reduction in symptoms of state anxiety (Mean±SD:47.1±12.2, Mean±SD:42.8±9.8, Mean±SD:41.7±9.1, respectively). At 1st month and discharge, were found to differ significantly only by maternal age, with fathers with younger partners (<33 years) having significantly more state anxiety symptoms compared with them with older partners (≥33 years). Furthermore, fathers’ state anxiety at discharge was significantly higher, in cases that mothers had conceived spontaneous compared with them who conceived under IVF treatment (p=0.027). At 1st month, father’s state anxiety score was significantly reduced at 1st month compared to 1st week, in cases with Apgar score at 5?minute<8. With the other variables being available in the table 6, no significant differences were found in the score of state anxiety in this assessment. Fathers’ scores of state anxiety were significantly reduced at 1st month and discharge compared to 1st week regardless of maternal age, length of hospitalization, birth weight, CRIB II score, Apgar score at 1? & 5?, and the presence or not of the mother’s health problem. Also, fathers’ score of state anxiety were significantly decreased at 1st month and at discharge compared to 1st week, in cases that the mother was primiparous and in those who conceived spontaneously. Fathers’ scores of state anxiety were significantly reduced from 1st week to discharge in cases with IVF conception. The degree of reduction in symptoms of state anxiety didn’t differ significantly with the variables in the table 6.
Mothers’ and fathers’ trait score at each assessment are presented in figure 1. Mean mothers’ trait score was 46.6 (SD=14.6) at 1st week, 41.9 (SD=14) at 1st month and 42.8 (SD=13) at discharge. Mean fathers’ trait score was 36.9 (SD=10.4) at 1st week, 36.6 (SD=9.7) at 1st month and 36.5 (SD=10.8) at discharge. At all assessments mothers’ score was significantly higher than fathers’. Also, mothers’ trait score at 1st week was significantly greater than at 1st month (p=0.001) as well as at discharge (p<0.001). Fathers’ trait score remained in similar levels across all assessments. Overall, the degree of trait score’s decreasing differed significantly between fathers and mothers, with mothers showing a decrease while fathers presenting a similar score during the follow–up (p=0.002).
|
|
|
Fathers’state anxiety |
P2 |
P3 |
||||
|
|
|
1st week |
1st month |
At discharge |
|
|
|
|
|
|
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
1stweek vs 1st month |
1St week vs at discharge |
1st month vs at discharge |
|
|
Total sample |
|
47.1 (12.2) |
42.8 (9.8) |
41.7 (9.1) |
<0.001 |
<0.001 |
>0.999 |
- |
|
|
<33 years |
48.6 (11.5) |
44.8 (9.5) |
43.9 (9.8) |
0.044 |
0.030 |
>0.999 |
0.458 |
|
Mothers’ age |
≥33 years |
45.5 (13) |
39.1 (9.4) |
39.2 (7.7) |
0.001 |
0.003 |
>0.999 |
|
|
P1 |
0.332 |
0.025 |
0.035 |
|
|
|
|
|
|
Primiparous |
No |
47.2 (12.3) |
43.4 (11.9) |
44.5 (9.7) |
0.225 |
0.802 |
>0.999 |
0.270 |
|
Yes |
47 (12.4) |
41.7 (9) |
40.6 (8.7) |
<0.001 |
<0.001 |
0.669 |
||
|
P1 |
0.969 |
0.552 |
0.134 |
|
|
|
|
|
|
Single pregnancy |
No |
44.8 (14.4) |
40.8 (9.8) |
40.8 (9.4) |
0.243 |
0.387 |
>0.999 |
0.688 |
|
Yes |
47.8 (11.6) |
42.5 (9.9) |
41.9 (9.1) |
<0.001 |
<0.001 |
>0.999 |
||
|
P1 |
0.430 |
0.564 |
0.714 |
|
|
|
|
|
|
Conception |
Spontaneous |
47.6 (11.1) |
42.9 (9.8) |
42.7 (8.9) |
0.001 |
0.002 |
>0.999 |
0.412 |
|
IVF |
44.2 (17.9) |
37.9 (9.1) |
35.4 (7.6) |
0.084 |
0.025 |
0.730 |
||
|
P1 |
0.455 |
0.161 |
0.027 |
|
|
|
|
|
|
Hospitalization duration |
<62 days |
47.8 (11.7) |
43 (9.1) |
41.9 (9.3) |
0.010 |
0.005 |
>0.999 |
0.825 |
|
≥62 days |
46.4 (12.9) |
41.3 (10.5) |
41.4 (9) |
0.005 |
0.017 |
>0.999 |
||
|
P1 |
0.653 |
0.501 |
0.793 |
|
|
|
|
|
|
Birth weight |
<1235 gr |
47.4 (11.9) |
43.4 (9.2) |
42.5 (9.1) |
0.043 |
0.022 |
>0.999 |
0.599 |
|
≥1235 gr |
46.8 (12.8) |
40.8 (10.3) |
40.8 (9.1) |
0.001 |
0.004 |
>0.999 |
||
|
P1 |
0.859 |
0.309 |
0.529 |
|
|
|
|
|
|
CRIB II SCORE |
<7 |
46.2 (13.3) |
40.9 (10.2) |
39.9 (9.2) |
0.006 |
0.004 |
>0.999 |
0.752 |
|
≥7 |
47.8 (11.5) |
43.1 (9.5) |
43.1 (8.8) |
0.008 |
0.020 |
>0.999 |
||
|
P1 |
0.626 |
0.395 |
0.204 |
|
|
|
|
|
|
Apgar score 1’ |
<7 |
48.9 (12.3) |
43 (9.7) |
43.5 (8.6) |
0.001 |
0.014 |
>0.999 |
0.560 |
|
≥7 |
45.5 (12.2) |
41.4 (10) |
40 (9.3) |
0.023 |
0.007 |
0.614 |
||
|
P1 |
0.287 |
0.547 |
0.150 |
|
|
|
|
|
|
Apgar score 5’ |
<8 |
49.1 (12.1) |
42.5 (10.6) |
44 (8.5) |
0.002 |
0.050 |
0.985 |
0.360 |
|
≥8 |
46 (12.3) |
41.9 (9.5) |
40.4 (9.2) |
0.010 |
0.002 |
0.385 |
||
|
P1 |
0.350 |
0.832 |
0.172 |
|
|
|
|
|
|
Mother’s health problem |
?χι |
47.5 (12.1) |
43.7 (9.6) |
42.2 (9.7) |
0.010 |
0.002 |
0.399 |
0.198 |
|
Ναι |
46.1 (12.9) |
38 (9.2) |
40.2 (7.4) |
0.001 |
0.041 |
0.719 |
||
|
P1 |
0.684 |
0.048 |
0.369 |
|
|
|
|
|
1p-value for group effect 2 p-value for time effect 3p-value from repeated measures ANOVA, regarding time*group effect

Figure 1: Mothers’ and Fathers’ Trait Score during Study Follow Up
Mothers’ and fathers’ state score at each assessment are presented in figure 2. Mean mothers’ state score was 52.2 (SD=11.9) at 1st week, 47.8 (SD=12) at 1st month and 49 (SD=10.3) at discharge. Mean fathers’ state score was 47.1 (SD=12.2) at 1st week, 42.1 (SD=9.8) at 1st month and 41.7 (SD=9.1) at discharge. At all assessments mothers’ score was significantly greater than fathers’. Also, for both mothers and fathers, state score at 1st week was significantly greater than at 1st month (p=0.013 for mothers and p<0.001 for fathers) as well as at discharge (p = 0.039 and p<0.001, respectively). From 1st month until discharge state score remained at similar levels for both parents. Overall, the degree of state score’s decrease was similar in both parents (p=0.310).
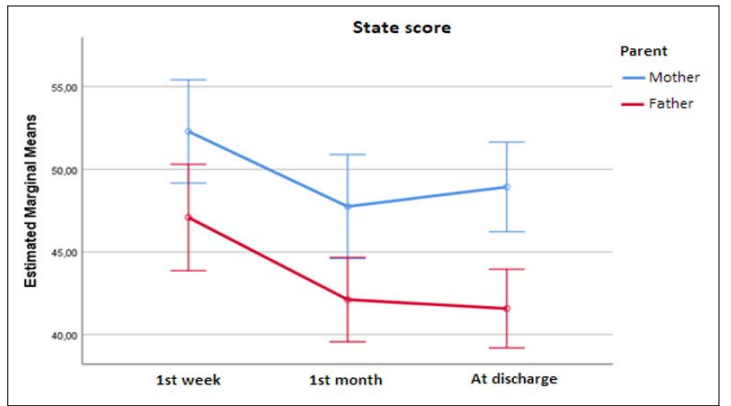
Figure 2: Mothers’ and Fathers’ State Score during Study Follow Up
Via linear regression it was found that fathers’ educational level was found to be significantly associated with the fathers’ trait score both during the 1st month (b=-6.55; SE=2.37; p=.008) and at discharge (b=-6.00; SE=2.11; p=.006). Specifically, higher educational level was associated with significantly fewer anxiety symptoms both at 1st month and at discharge. With fathers’ state score at discharge, their educational level (b=-4.35; SE=1.98; p=.032) and mother’s age (b=-4.13; SE=2.11; p=.050) were found to be significantly associated.
Specifically, higher parental educational level was associated with significantly fewer anxiety symptoms at discharge. Also, when the newborn’s mother was at least 33 years old, fathers had a 4.13-point lower score, meaning significantly fewer symptoms of state anxiety at discharge. Only the pre–pregnancy weight was found to be significantly associated with mothers’ trait score at discharge (b=0.26; SE=0.13; p=.047). Specifically, greater pre- pregnancy weight was associated with significantly more anxiety symptoms at discharge.
Preterm birth is an important issue in public health. In recent decades, the rapid progress in perinatal medicine have improved the treatment of preterms in NICUs, leading to a greater survival rate, reduced mortality but increased morbidity [35]. The increased survival rate of preterms leads to a long stay in NICU that requires control of parental feelings because they will be involved in the care of their children but also, after the discharge of the hospital [36-38]. Parental feelings in NICU are often a neglected area and much of the caregiving in this environment experienced by the infants as medical intervention, as a result the NICU is often infant- centred instead of family-centered. Last decades, researchers focused on depression in parental perinatal mental health and less clinical and research attention had been paid to parental perinatal anxiety. Recent studies have shown that parents of preterm infants are at high risk for developing anxiety symptoms. Previous study found that among NICU parents, 41.9% reported experiencing anxiety and 39.9% post-traumatic stress. Nowadays, the need for further studies on promoting the parental mental health is becoming increasingly apparent [5-45].
This study sought to determine the intensity of anxiety experienced by parents in the NICU. Anxiety was found to be quite relevant to this population. Specifically, our findings indicated that scores measuring a feeling of anxiety was highest on STAI scales, suggesting that anxiety experienced by parents is high shortly after birth. This design allowed us to capture parents at the critical fragile time-point within a consistently small timeframe postpartum (7 days). Parents experienced the preterm birth as a stressful event that could increase stress and negative feelings such as anxiety, anger and depression. Our findings corroborated by the results of older studies, which also showed that parents of hospitalized preterm infants are at high risk to develop anxiety and depressive symptoms. NICU seems scary for parents due to the environmental conditions such as staying in an incubator, monitoring, noisy life support system and bright lights. Also, invasive procedures and constantly changing healthcare professionals increase the risk for parental anxiety even more [5-49].
Another interesting finding was that feelings of anxiety improved during the infant’s hospitalization and especially just before the discharge of the NICU. Similarly, recent studies concluded that parents of preterm infants had elevated rates of anxiety symptoms that declined during the newborn period and especially at discharge [50,51]. Another study, reported that the proportions of both parents with elevated anxiety symptoms was also initially high, approaching 50% for both. Also, throughout the newborn period, the rate of mothers with elevated anxiety never dropped below 22%, while for fathers the lowest rate was 25% [15]. We hypothesised that the improved of parents’ psychological state wasn’t only due to the recovery of their infants but also, to the support and care they receive from the NICU staff. Our hypothesis corroborated by previous studies, which showed that the interaction between staff of NICU and parents would be essential in helping them cope with their infants’ NICU stay [52-55].
Our findings underlined that mothers experienced more mental and physical challenges than did fathers during the NICU stay, a finding consistent with previous studies [47,56-58]. Moreover, mothers had significantly higher anxiety scores for each sub-scale compared with fathers (p<0.002). Preterm birth has been reflected negatively on parents’ psychological wellbeing, causing symptoms such as anxiety, depression and post-traumatic stress disorder [5,16,42,59]. According to previous study, mothers’ experience of greater anxiety may be due to more negative appraisals of their infants’ [60]. On the other hand, fathers employ more effective coping strategies to decrease anxiety. Probably, mothers perceived their infants’ prematurity as more difficult than fathers, at admission and discharge. Similar results were found by three years later. Whereby more mothers than fathers were worried about their infants’ future difficulties regarding development and health at discharge. Also, mothers’ increased anxiety, the first days of their infants’ NICU admission, may be caused by mood disturbances from the premature birth of their infants through “baby blues”, postpartum mood disorders or obstetric procedures. The above suggests that mothers and fathers have expectations for their role in caring their infants during the NICU stay [61]. In contrast, previous study presented different anxiety patterns with fathers of preterm infants having overall higher anxiety scores of mothers [62]. Similarly, showed that the difference in the prevalence of anxiety between mothers and fathers was statistically significant (6.6% for mothers vs 21% for fathers, p<0.01). Also, other researchers failed to identify group differences between mothers and fathers of preterm infants [63-66]. So, the gender variable wouldn’t be affecting the parents’ interaction with their infants.
The age of mother is one of the factors that affects the state-trait anxiety of parents during this period. Our study showed that mothers’ trait anxiety levels was significant decreased in women who were at a least 33 years old at three assessments (p=0.004; p<0.001, respectively). Our findings corroborate the results of older studies, which also concluded that mothers’ stress levels increased with increasing age after preterm birth and admission of their infant to NICU [42,67]. Contrasting, previous studies found that higher anxiety levels were significant associated with age groups in the rage of younger mothers with preterm infants in NICU [68-70]. Furthermore, maternal age was a significant predictor of anxiety. In particular, younger mothers reported higher levels of anxiety [49]. According to previous research, women with advanced age had adverse obstetric outcomes more often than did younger women [71]. So, we hypothesised that this population is at increased risk from the beginning of pregnancy for complications and may has been managed or prepared psychologically to deal with them. Whereas other studies didn’t find association between maternal age and high levels of anxiety [66,71].
Another interesting finding in this study was the mothers’ age were found to be significant associated with fathers’ state score at discharge. Specifically, when mothers’ age was at least 33 years old, fathers had a 4.13-point lower score, means fewer symptoms of state anxiety at this assessment. Also, regarding time group effect from repeated measures ANOVA was found that the degree of change in scores of fathers’ trait anxiety differed significantly only according to the maternal age (p=0.007). Simultaneously with the developmental stages of individuals, the family’s life cycle and social conditions, the roles of family members are differentiated and changed [37]. Thus, partners/fathers after premature birth change their role according to their mothers’ needs, acquiring a supportive role. They are expected to help their partners in coping with immaturity due to their age, negative feelings, such as anxiety, depression and sufferance, and think about their family, giving them proper support [72,46].
Also, a recent study explored how the fathers experienced their role as a support for their partner ant the relationship with them during their NICU stay, found that 85% of fathers had put aside their own needs for support, suppressing their feelings and relaxation to take care their partners and infant, but this doesn’t mean that they weren’t in need of attention and care. In cases where the mothers’ situation was severe after childbirth, they were more concerned about them than their newborn, while the mothers themselves were more concerned about the newborn. The presence of fathers next to mothers and newborns in the NICU was beneficial for the mothers, who reported experiencing increased stress when they were alone [73-75].
Our exploratory findings suggested that fathers’ scores of state anxiety were significantly reduced at 1st month and discharge compared to 1st week, in cases of Apgar score 5? <8. It was observed that the time of seeing the infant for first time was also effective on the anxiety and stress of parents [76]. Parents and especially fathers are the ones who see their infants within the first half an hour as the majority of mothers underwent cesarean section and remained bedridden. This meeting shortly after birth, once infants have been received medical care, confronts fathers with the criticality of their infants’ condition. Besides that, according to the scientific community the combination low Apgar score at 1 and 5 minutes, especially when both scores are low, is associated with long-term neurological morbidity [77]. This can have a significant impact on fathers’ psychological wellbeing.
Our results failed to identify correlation between duration of infants’ hospitalization and parental anxiety. It has been shown that even for a very short period stay of infants in the NICU causes crisis in families [78]. These findings weren’t corroborated by the results of other studies, which showed that the extension of the hospitalization significantly affected the stress and anxiety of the parents of preterm infants [5,79,80]. In particular, the days of hospitalization correlated positively with various felling, such as anger, anxiety, avoidance, hostility, and depression [5]. Also, a recent study found a positive correlation between the length of NICU stay of infants and the mothers’ stress scale and the parental role [12].
Our study showed that parents of infants who were conceived spontaneously experienced higher levels of anxiety (mothers’ trait & fathers’ state) compared to those who conceived by in-vitro fertilization (IVF). In contrast, previous studies concluded that women who underwent IVF experienced higher levels of state anxiety. As is common knowledge, these women subjected to a variety of diagnostic procedures and tests, which aren’t always initial successful and likely leading to anxiety and disappointment [42,81]. While, the couple who conceive spontaneously don’t experience all the emotional distress of trying and often failing. They also, dream of a healthy and “perfect” baby without being prepared for the occurrence of problems during pregnancy or unexpected event of a premature birth. As a consequence, they are being unprepared for the possibility of complications in their pregnancy and childbirth, leaving them mentally vulnerable.
Another interesting finding was that only the pre-pregnancy weight was significantly associated with mothers’ trait score at discharge. Specifically, advanced pre-pregnancy weight was associated with significantly more anxiety symptoms. Appearance is closely associated with person’s self-esteem, particularly in women, and a low self-esteem is a risk factor for depression and consequently anxiety. A systematic review concluded that the majority of studies suggested that obesity pregnant women may be a subgroup that is particularly vulnerable to anxiety and in need of targeted psychological support [82].
Another variable examined was the fathers’ educational level and the anxiety scores. Specifically, higher educational level was associated with fewer anxiety symptoms during study period. Similarly, recent studies showed that fathers with lower education level had more anxiety symptoms. Other studies didn’t find a correlation between fathers’ demographic characteristics and anxiety levels [63,66,83]. The above data can’t constitute a generalized rule, due to the lack of several studies concerning the emotional state of fathers in the perinatal period, which requires further analysis.
Our study has several limitations that have to be mentioned. Initially, this study conducted during the pandemic of Covid-19, so the recruitment of parents was difficult for the researcher due to the intensity of the NICU and hospital in Greece. Also, the studied group was parents with preterm infants, who were vulnerable, need specialised care and some of them didn’t survive. Furthermore, need support from equipment that wasn’t often available to cover their hospitalization, as a consequence they transferred to other NICUs. All these factors have reduced the population of present study because our communication interrupted and excluded of the study. Finally, the self-reported instruments used to the study constitute a great resource for psychological research but using them solely can’t ensure an objective conclusion.
As a consequence of the current study, it was determined that parents with preterm infants perceived high levels of anxiety during the NICU stay. The severity of parental anxiety (state & trait) was influenced by various sociodemographic factors of them and simultaneously various infants’ clinical characteristics. Premature birth undoubtedly has a significant impact on mental and emotional state of parents and especially mothers. In conclusion, the scientific community should run further research targeted to the factors that influence the parental mental health, such as personality traits, mental health issues and the interaction with the NICU’s staff.
Additionally, should recommend establishing perinatal interdisciplinary teams of health specialists (e.g, doctors, midwives, nurses, psychologists) and interventions to support the new families in deferent domains of their life (interpersonal, family, individual, social and professional).
The authors declared no potential conflicts of interest with respect to the research, authorship and publication of this article.
