Author(s): <p>Dakshata Atare, Tejaswini Shekhar Sharma, Edavally Sainath Reddy, Krupa Ajmera Modi* and Sharmila Patil</p>
Verruca Vulgaris or common warts are benign proliferative hyperkeratotic growths caused by infection with the human papilloma virus (HPV). Warts are common in humans and account for 8% of visits to dermatologists. Although the spontaneous resolution rate for warts is 65-78% within two years, the cosmetic disfigurement, tendency to spread and associated poor quality of life warrants quick intervention
Verruca Vulgaris or common warts are benign proliferative hyperkeratotic growths caused by infection with the human papilloma virus (HPV). Warts are common in humans and account for 8% of visits to dermatologists. Although the spontaneous resolution rate for warts is 65-78% within two years, the cosmetic disfigurement, tendency to spread and associated poor quality of life warrants quick intervention [1, 2].
There are many destructive therapies available for the treatment of common warts, and no single treatment has yet proven 100% effective. Destructive therapies include either topical agents, such as salicylic acid, podophyllotoxin, trichloroacetic acid, formaldehyde, 5- fluor-ouracil and photodynamic therapy; surgical methods such as cryosurgery, electrocautery, and surgical excision. Immunomodulating agents include contact sensitizers, imiquimod, intrale-sional interferons and oral drugs like levamisole, cimetidine, and zinc sulphate [3, 4].
The mounting evidence that cell-mediated immunity (CMI) plays a major role in wart resolu-tion highlights the need for immune protection against HPV. This observation has directed the attention towards stimulation of patients? immune system, particularly CMI, to eradicate the virus. Based on this assumption, various antigenic stimulants of CMI have been studied in recent years. The various agents used for intralesional Immunotherapy include candida anti-gen, mumps antigen, Trichophyton skin test antigen, tuberculin antigen, BCG vaccine, MMR vaccine, Mycobacterium w vaccine [5-11].
Intralesional Immunotherapy has the potential advantages of clearance of both treated and untreated distant warts without scarring, a presumed lower rate of recurrence and a high safe-ty profile [5, 6].
There are many agents used for intralesional Immunotherapy with variable results in terms of safety and efficacy. As MMR vaccine is included in the vaccination schedule, all patients are expected to be immune to it, which helps in mounting a stronger immune response to the MMR vaccine injected intralesionally [14]. In addition, the presence of three different anti-gens in the MMR vaccine makes the probability of sensitivity to the injected antigen very high and the likelihood of anergy to the three antigens extremely low [8].
Also, the MMR vaccine has a better side effect profile with tolerable pain at the site during injection and mild flu-like symptoms being the most common side effects. Ulceration and lymphadenitis necessitating antituberculous therapy following the use of BCG as the immu-notherapeutic agent are reported. Rare adverse events like post- Immunotherapy revealed cicatrix (PIRC) and painful purple digit could develop following use of candida antigen. Thus, taking advantages of these observations, we designed our present study to evaluate the efficacy and safety of the MMR vaccine in the treatment of common warts [13-15].
The present prospective uncontrolled study was conducted in the Department of Dermatolo-gy, Venereology and Leprosy. After ethical approval and informed consent, a total of 100 patients was enrolled as per inclusion-exclusion criteria. Patients with single or multiple warts of age more than 18 years with no concurrent systemic or topical treatment of warts within the past 8 weeks were included in the study. However, patients with Genital warts, age less than 18 years, pregnancy, lactation, Immunosuppression, patients with fever or signs of any inflammation or infection, the patient who had received any other treatments for their warts in the last two months before enrolment and past history of asthma, allergic skin disorders and patients with meningitis or convulsions were excluded.
Detailed demographic data of each participant were collected. The detailed cutaneous exami-nation was conducted in bright light, and appropriate digital photographs were taken at base-line and every three weeks. The MMR vaccine was available in the form of single-dose vial of freeze-dried vaccine. It was reconstituted with 0.5 ml of diluent (water for injection). The reconstituted vaccine can be stored at 2-8 degree Celsius for 6 hours. On day 1, 0.1- 0.3 ml of the reconstituted vaccine was injected intralesionally in the largest or the oldest wart using an insulin syringe. After the injection, the patients were followed up after 48 hours for pain and inflammation; in case of severe inflammation, NSAIDS were given if required. The treatment was repeated every three weeks till complete remission or for a maximum of five sessions without response. After completion of the treatment schedule, the patients were fol-lowed every month for three months for clinical assessment of results and recurrence. Find-ings obtained were documented in a specially prepared proforma and photography. At the end of the study period, pre-and poet- intervention photographs were assessed to compare the degree of reduction of the wart. The response was evaluated as follows: Grade 1- No re-sponse; Grade 2- 0%- 49% reduction in size; Grade 3- 50%- 99% reduction in size; and Grade 4- Complete Response (disappearance of lesions with the appearance of normal skin markings).
Qualitative data was presented using frequency, percentage and Quantitative Data was pre-sented using descriptive statistics such as Mean, SD, and SEM. Further statistical analysis was carried out with the help of statistical tests such as the Z- test for proportion and the chi-square test for association. Level of significance was set at 5%. All p- values less than 0.05 was treated as significant.
In our study, the majority (71/107; 66.36%) of patients belonged to the 21-40 years age group, 24 (22.43%) were < 20 years of age, and the rest 12 (11.21%) were >41 years. Most of the patients were male(73/107; 68.22%) as compared to 34 (31.78%) females. Most (48/107; 44.86%) patients had warts from 6-12 months, 32 (29.91%) had warts from 12-24 months, 22 (20.56%) had warts from <6 months, while only 5 (4.67%) had warts for >24 months [Table-1]. With respect to the distribution of the type of warts, palmoplantar warts and Verruca Vul-garis warts were observed in 40/107 (37.38%) patients each; 16 (14.95%) patients had periungual warts, and 11 (10.28%) had verucca plana warts [Figure-1].
| No | % | ||
|---|---|---|---|
| Duration | <6 months | 22 | 20.56 |
| 6 to 12 months | 48 | 44.86 | |
| 12 to 24 months | 32 | 29.91 | |
| >24 months | 5 | 4.67 | |
| Number of warts |
Multiple | 73 | 68.22 |
| Single | 34 | 31.78 | |
| Presence at distant site |
Yes | 57 | 53.27 |
| No | 47 | 43.93 | |
| Response | Partial response | 16 | 14.95 |
| Complete response | 82 | 76.64 | |
| No response | 9 | 8.41 |
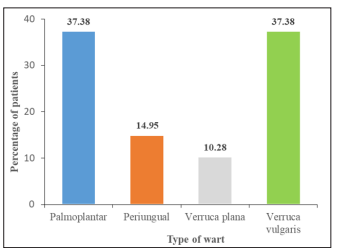
Figure 1: Graphical representation as per type of wart in patients
Among our study population, a majority, i.e. 73/107 (68.22%) patients had multiple warts, whereas 34 (31.78%) patients had single warts. The presence of the warts in the main sites in our study population was as follows: 23 (21.50%), face; 21 (19.63%), soles; 18 (16.82%), palms; 17 (15.89%), hands; 14 (13.08%), nails; 13 (12.15%), legs; 2 (1.87%), scalp and 1 (0.93%), nasal mucosa [Figure-2]. Apart from the main site, additional warts were observed at a distant site in 57 (53.27%) patients. Most patients were students (36; 33.64%) followed by those with office job (31; 28.97%), 18 (16.82%) were housewives, 10 (9.35%) were labourers, while the rest, 12 (11.21%) had other occupations.
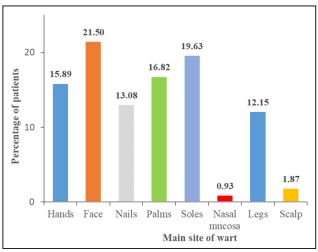
Figure 2: Graphical representation as per main site of wart in patients
Out of 107 patients, 38 had taken prior treatment for warts: 20 were treated with a combina-tion of salicylic acid and lactic acid, 14 had undergone electrocautery, two patients were treated by parring or with the use of oral zinc and tretinoin cream and one patient was treated with trichloroacetic acid. The median number of sessions needed by our patients was 5, with a minimum of 1 session to a maximum of 5 sessions.
Recurrence of warts was observed in 2 (1.87%) patients, whereas in the majority, i.e. 105/107 (98.13%) patients, no recurrence was seen. A complete response to our treatment was ob-served in a majority (82/107; 76.64%) of patients. Partial response was noted in 16 (14.95%) patients, whereas no response was recorded in 9 (8.41%) patients. Majority (87; 81.31%) did not have any side effects. However, the following distribution of side effects was noted in the rest 21 patients: 14 (13.08%) reported pain, 6 (5.61%) had itching, and 1 (0.93%) patient had erythema [Figure-3].
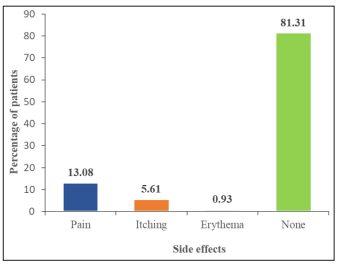
Figure 3: Graphical representation as per side effects in wart patient
Of the 16 patients that showed partial response, 4 (25%) had warts since <6 months, 9 (56.25%) patients had warts since 6-12 months, 3 (18.75%) had warts since 13-24 months, while none had warts since >24 months. Of the 82 patients that showed complete response, 44 (53.66%) had warts since <6 months, 34 (41.46%) patients had warts since 6-12 months, 26 (31.71%) had warts since 13-24 months, and 4 (4.88%) had warts since >24 months. Of the 9 patients that showed no response, none had warts since <6 months, 5 (55.56%) patients had warts since 6-12 months, 3 (33.33%) had warts since 13-24 months, and 1 (11.11%) had warts since >24 months [Table-2, Figure-4].
Out of 16 patients that showed partial response, 4 (25%) had palmoplantar warts, 3 (18.75%) had periungual warts, 2 (12.5%) had verruca plana warts, and 7 (43.75%) had Verruca Vul-garis warts. Of the 82 patients that showed complete response, 34 (41.46%) had palmoplantar warts, 11 (13.41%) had periungual warts, 8 (9.76%) had verruca plana warts, and 9 (10.97%) had Verruca Vulgaris warts. Of the nine patients that showed no response, 2 (22.22%) pa-tients each had the palmoplantar and periungual type of warts, 1 (11.11%) patient had Verru-ca plana warts, and 4 (44.44%) had Verruca Vulgaris warts. The comparison between the group was made using the chi-square test, which revealed no statistically significant differ-ence (p=0.10365) in the distribution of the different types of warts among the three groups [Table-2, Figure-4].
Among the 16 patients that showed partial response, 1 (6.25%) patient had a single site af-fected as compared to 15 (93.75%) who had warts at multiple sites. Among the 83 patients that showed complete response, 16 (19.51%) had a single site affected, whereas 66 (80.49%) had warts at multiple sites. Among the 9 patients that showed no response, 2 (22.22%) were affected at a single site as compared to 7 (77.78%) that had warts at multiple sites. The comparison between the group was made using the chi-square test, which revealed no statistically significant difference (p=0.4176) in the distribution of the number of sites among the three groups [Table-2, Figure-4].
The need for the number of sessions for treatment between the groups was compared and recorded. As compared to 4 sessions (range 1-5) needed by the partial response group, five sessions were required by all patients to achieve a complete response, and even after five ses-sions, nine patients did not show any response.
| Parameter | Type of response | p-value* | ||
|---|---|---|---|---|
| Partial (n=16) | Complete (n=82) | None (n=9) | ||
| Duration of warts, N (%) <6 months 6-12 months 13-24 months >24 months |
4 (25) 9 (56.25) 3 (18.75) 0 |
44 (53.66) 34 (41.46) 26 (31.71) 4 (4.88) |
0 5 (55.56) 3 (33.33) 1 (11.11) |
|
| Type of warts, N (%) Palmoplantar Periungual Verruca plana Verruca vulgaris |
4 (25) 3 (18.75) 2 (12.5) 7 (43.75) |
34 (41.46) 11 (13.41) 8 (9.76) 9 (10.97) |
2 (22.22) 2 (22.22) 1 (11.11) 4 (44.44) |
0.10365 |
| Number of sites, N (%) Single Multiple |
1 (6.25) 15 (93.75) |
16 (19.51) 66 (80.49) |
2 (22.22) 7 (77.78) |
0.4176 |
| No. of sessions, median (min, max) | 4 (1, 5) | 5 (5, 5) | 5 (5, 5) | - |
| *Calculated using the chi-square test. P<0.05 considered statistically significant | ||||

Figure 4: Clinical picture of wart patients as per their responsiveness
The present study was designed to assess the efficacy of intralesional Immunotherapy in warts using MMR vaccine in a tertiary care centre in Navi Mumbai. In our study, of the 107 patients, complete response to the MMR intralesional treatment was observed in a majority, i.e. 82 (76.64%) of patients, partial response was noted in 16 (14.95%) patients; however, no response was recorded in 9 (8.41%) patients. In a similar study conducted by Raju J et al.,[12]. among 30 patients with warts, complete remission was noticed in 19 (70.4%) cases, partial remission in 6 (22.22%) cases, and no response was seen in 2 (7.4%) cases. Nofal et al [5]. conducted a controlled study to assess the efficacy of the MMR vaccine in recalcitrant warts in 65 patients. They found the complete response to treatment in 41 (63%) patients, par-tial response in 15 (23%) patients and no response in 9 (14%) patients. Na CH et al [15].
observed complete response in 26.5% of their study patients, which was followed by the par-tial response in 25%, while no response was seen in 48.5% of patients. In the 50 patients treated with MMR by Mohamad NS et al [16].complete response was seen in 41 (82%) pa-tients, partial response in 3 (6%) patients and no response in 6 (12%) patients. The results of our study (76.64% complete response) were higher than those reported by Kus et al [17]. (29.4%) and Clifton et al [18].(47%), who have used intralesional Immunotherapy (tuberculin and mumps or candida, respectively). The relatively higher response in our study which was comparable with other studies using MMR vaccine-like Mohammad NS et al [16]. and Nofal et al [10]. maybe attributed to the presence of three viral antigens in the MMR vaccine, which could lead to a stronger stimulation of the immune system. Moreover, viable vaccines have been considered to be more immunogenic than the skin test antigens like candida and tuber-culin [11].
In our study, the majority (71/107; 66.36%) patients belonged to the 21-40 years age group, 24 (22.43%) were <20 years of age, and the rest 12 (11.21%) were >41 years. Raju J et al [12]. observed 63% of his study population was between 20-40 years of age. The population studied by Nofal et al. [7] were in the 18 to 55-year age group, while that in the Na CH et al. [15] study ranged from 3 to 64 years. Most of our study population (73/107; 68.22%) were males compared to 34 (31.78%) females. Similar male gender predominance was noted by Raju J et al [12]. (62.9%), Nofal et al. [10] (35/65; 53.85%) and Na CH et al [15]. (61.8%). Male predominance is seen may be due to the outdoor working condition common in the male gender.
In the present study, most (48/107; 44.86%) patients had warts from 6-12 months, 32 (29.91%) had warts from 12-24 months, 22 (20.56%) had warts from <6 months, while only 5 (4.67%) had warts for >24 months. In a similar study by Na CH et al [15]. majority (39%) patients had warts for >24 months, 26.5% patients had warts for <6 months and between 12-24 months, while 8.1% patients had warts for <6 months. In the study by Raju J et al [12]. in 66.6% of patients, the duration of warts was <1 year. The duration of lesions observed by Nofal et al [10]. ranged between 2 to 15 years.
With respect to the distribution of the type of warts noted in our study, palmoplantar warts and Verruca Vulgaris warts were observed in 40/107 (37.38%) patients each; 16 (14.95%) patients had periungual warts, and 11 (10.28%) had Verucca plana warts. Common warts were the most predominant (40/65; 61.54%) type observed by Nofal et al [10]. The distribu-tion of the type of wartsas observed by Na CH et al [15]. Was: common warts (47.8%), palmoplantar warts (47.1%) and verruca plana (5.1%).
Among our study population, a majority, i.e. 73/107 (68.22%) patients had multiple warts, whereas 34 (31.78%) patients had single warts. Most studies have reported the presence of multiple warts in most patients. Na CH et al [15]. reported multiple warts in 90.4% of pa-tients; single warts were seen in 9.6% of patients. Multiple warts were observed in all patients studied by Raju J et al [12]. And Nofal et al [10].
The presence of the warts in the main sites in the present study population was as follows: 23 (21.50%), face; 21 (19.63%), soles; 18 (16.82%), palms; 17 (15.89%), hands; 14 (13.08%), nails; 13 (12.15%), legs; 2 (1.87%), scalp and 1 (0.93%), nasal mucosa. Raju J et al [12]. re-ported hand and foot involvement in 25 (92.6%) patients, while face and neck involvement was seen in 2 (7.4%) patients. Face, palms and sole involvement was commonly seen in our study. This may be because of trauma, pricks, and inoculation; also, walking bare feet- a prac-tice common in Indians may attribute to the increased incidence of plantar warts.
Amongst our study patients, apart from the main site, additional warts were observed at a distant site in 57 (53.27%) patients. Similar findings have been described by various authors. In the study by Nofal et al [5]. Distal warts were noted in 51 out of 65 patients. Distant warts were observed in 66.7% of patients by Na CH et al [15].
Out of 107 patients, 38 had taken prior treatment for warts: 20 were treated with a combina-tion of salicylic acid and lactic acid, 14 had undergone electrocautery, two patients were treated by parring or with the use of oral zinc and tretinoin cream and one patient was treated with trichloroacetic acid. In the study by Na CH et al [15]. previous treatment was taken by 60.3% of patients. In our study, the median number of sessions needed by our patients was 5, with a minimum of 1 session to a maximum of 5 sessions. On similar lines, in the study by Nofal et al [5]. An average of 5 sessions were needed by most patients.
Recurrence of warts was observed in 2 (1.87%) patients, whereas in the majority, i.e. 105/107 (98.13%) patients, no recurrence was seen. Similar results were obtained Nofal et al [5]. wherein recurrence after complete clearance at the 6-month follow-up was seen in 2/65 (4.8%) patients. Raju et al [12]. observed no recurrence in their complete remission group. Thus, re-duction or prevention of recurrences is an important advantage of Immunotherapy over the destructive and often painful treatment modalities. This may be the result of the acquisition of immunity to HPV by induction of CMI that stimulates the production of memory T cells against the virus leading to a strong effector response [18].
Amongst our study population, the majority (87; 81.31%) did not have any side effects. However, the following distribution of side effects was noted in the rest 21 patients: 14 (13.08%) reported pain, 6 (5.61%) had itching, and 1 (0.93%) patient had erythema. The pain was reported during injection, which did not last for more than 10 minutes in all 21 patients. Similar findings were reported in various similar studies. Raju et al [12]. And Mohamad NS et al [16]. Reported pain during injection and flu-like symptoms in 2 out of 30 patients. The distribution of the various complications observed by Nofal et al [10]. among his study popu-lation included: pain (100%), flu-like symptoms (12.3%), itching (6.1%), erythema (4.6%) and oedema (1.5%) [7].
The more aggressive adverse effects of the traditional destructive modalities like scarring and pigmentary changes were not observed in our as well as other related studies, a significant advantage of Immunotherapy over the conventional methods of treatment [18].
Out of 107 patients, 16 patients showed partial response, 4 (25%) had warts since <6 months, 9 (56.25%) patients had warts since 6-12 months, 3 (18.75%) had warts since 13-24 months, while none had warts since >24 months. Of the 82 patients that showed complete response, 44 (53.66%) had warts since <6 months, 34 (41.46%) patients had warts since 6-12 months, 26 (31.71%) had warts since 13-24 months, and 4 (4.88%) had warts since >24 months. Of the 9 patients that showed no response, none had warts since <6 months, 5 (55.56%) patients had warts since 6-12 months, 3 (33.33%) had warts since 13-24 months, and 1 (11.11%) had warts since >24 months. Na CH et al. [15] reported no statistically significant association be-tween the disease duration and response to treatment. A better cure rate in those with shorter disease duration was reported by Mohamad NS et al [16].
As per distribution of the type of warts was a concern, 16 patients showed partial response, 4 (25%) had palmoplantar warts, 3 (18.75%) had periungual warts, 2 (12.5%) had verruca plana warts, and 7 (43.75%) had Verruca Vulgaris warts. Of the 82 patients that showed complete response, 34 (41.46%) had palmoplantar warts, 11 (13.41%) had periungual warts, 8 (9.76%) had verruca plana warts, and 9 (10.97%) had Verruca Vulgaris warts. Of the nine patients that showed no response, 2 (22.22%) patients each had the palmoplantar and periungual type of warts, 1 (11.11%) patient had verruca plana warts, and 4 (44.44%) had Verruca Vulgaris warts. We observed no statistically significant difference (p=0.10365) in the response of the different types of warts among the three groups. Na CH et al. [15] reported a significantly higher clinical response of common warts (including periungual and subungual warts) than the other wart types (p < 0.05).
As per distribution of the number of sites was a concern, 16 patients showed partial response, 1 (6.25%) patient had a single site affected as compared to 15 (93.75%) who had warts at multiple sites. Among the 82 patients that showed complete response, 16 (19.51%) had a sin-gle site affected, whereas 66 (80.49%) had warts at multiple sites. Among the 9 patients who showed no response, 2 (22.22%) were affected at a single site compared to 7 (77.78%) who had warts at multiple sites. We observed no statistically significant difference (p=0.4176) in the distribution of the number of sites among the three response groups. Mohamad NS et al. [16] reported a significantly better response in multiple lesions than single ones. Na CH et al. [15] reported no statistically significant association between the number of warts and response to treatment.
Treatment of warts using the intralesional MMR technique appears better tolerated and a safe option as compared to the traditional destructive methods. As seen earlier, we report the oc-currence of warts to be more predominant in the younger generation, with a higher incidence in males. The complete response with no recurrence after a 6-month follow-up demonstrates the efficacy of the treatment. Future studies, including randomized controlled prospective trials, are needed to investigate the clinical effects and factors affecting the efficacy of this treatment.
