Author(s): Arjun Khanna*, Deepak Talwar, Linija K Nair, Kartik Deshmukh, Dhruv Talwar and Sourabh Pahuja
There were an estimated 37.9 million patients with asthma in India in 2016, with a crude prevalence of 2.9% [1]. Severe asthma is thought to affect approximately 5 – 10% of patients with asthma [2]. Omalizumab, a humanized recombinant monoclonal antibody against IgE, is the first biologic therapy approved for moderatesevere asthma. Omalizumab binds free IgE on the serum and leads to downregulation of FceR1 receptors on basophils and mast cells [3]. The safety and efficacy of Omalizumab have been demonstrated in multiple studies worldwide [4].
There were an estimated 37.9 million patients with asthma in India in 2016, with a crude prevalence of 2.9% [1]. Severe asthma is thought to affect approximately 5 - 10% of patients with asthma [2]. Omalizumab, a humanized recombinant monoclonal antibody against IgE, is the first biologic therapy approved for moderatesevere asthma. Omalizumab binds free IgE on the serum and leads to downregulation of FceR1 receptors on basophils and mast cells [3]. The safety and efficacy of Omalizumab have been demonstrated in multiple studies worldwide [4].
Omalizumab was approved in India in 2008, and a few studies have evaluated the safety and efficacy in the Indian population [5, 6]. However, we lack evidence regarding the real-world clinical characteristics and outcomes of patients on omalizumab therapy in India. The strict inclusion and exclusion criteria of randomized controlled trials do not always reflect real-world patients who often have comorbidities complicating the management. Hence, we designed the present study to understand the characteristics of patients who initiate Omalizumab in a real-world setting andunderstand the long-term outcomes of Omalizumab in India.
This was a retrospective, observational, cohort study using the data from health records of a tertiary care center in north India. Data were collected between June 2018 and May 2020.
We included patients aged 18 years or over with IgE mediated asthma (IgE levels 30-1500 IU/ml) who were prescribed omalizumab therapy for severe asthma refractory to high-dose ICS-LABA at MCRD Severe Asthma Clinic for a minimum duration of 16 weeks. Sixteen weeks cut-off was selected to identify omalizumab-responders and exclude non-responders from the study. Patients were evaluated at 16 weeks (±2 weeks) and 52 weeks(±4 weeks). Diagnosis of severe asthma was according to the GINA 2018 criteria [7]. Patients gave written informed consent, and the study was approved by the institutional review board (Ethics review board, Metro Hospital, and Heart Institute, Noida). Since most patients were elderly(>60 years), we meticulously reviewed the records to exclude patients with ACO-like phenotypes(persistent airflow limitation with clinical features consistent with both Asthma and COPD).
Data was collected retrospectively from institutional database and patients’ clinical records. Data during the pre-omalizumab period was collected from an institutional database, paper-based records, and in some cases, by requesting the general practitioners and referring hospitals. Patients underwent skin prick (antigens obtained from All Cure Pharma Pvt. Ltd and AllergoPharma GmbH & Co. KG) and/or specific IgE testing (ImmunoCAP, ThermoFisher Scientific); specific allergens were documented. Exhaled nitric oxide(FeNO) was measured at baseline ( Medisoft exhaled nitric oxide analyzer).
Previous prescriptions were used to assess the medications prescribed to patients for asthma at baseline. The age of onset of asthma was also inquired. Though there is no consensus on age cut-off to classify early-onset asthma and late-onset asthma (LOA), we considered patients to have late-onset asthma if they presented after 40 years [8]. Respective departments at our center evaluated comorbidities; we considered a patient to have specific comorbidity if the same was mentioned on the record (no confirmation was sought based on the current guidelines for the diagnosis of respective comorbidity).
Retrospective data on exacerbations, hospitalizations/emergency visits, medications, asthma control test (ACT) score, and lung function before initiation with omalizumab therapy was available for all patients included in the study
We excluded patients with insufficient baseline data. After initiation with omalizumab therapy, the data was captured as available during the patient visits to the center or on the phone calls by the healthcare staff. We tracked the prescriptions for medications prescribed for asthma; these include oral medications such as Leukotriene receptor antagonists, mucolytics, and inhaled medications such as ICS, ICS-LABA, LAMA-LABA-ICS, Betaagonists. To investigate the effect of Omalizumab on small airway function, we included a subset of patients subjected to impulse oscillometry (performed using Jaeger MasterScreen IOS)
The data was anonymized, and the staff involved in data analysis was trained in the study documentation requirements and reporting of adverse events.
The primary outcome was the annual rate of asthma exacerbations (defined as worsening of asthma symptoms requiring treatment for three days or more with systemic steroids or hospitalization or emergency visit) at the end of 52 weeks. The rate of asthma exacerbations over 52 weeks was further stratified according to baseline AEC (<300 or ≥300 per cubic millimeter), FeNO (<20 or ≥20 ppb), and IgE level (30-250, 251 - 699, ≥700 IU/ml).
Secondary outcomes included Asthma control test (ACT), Hospitalizations/emergency visits, OCS bursts, medications for asthma consumed per day, and lung function tests (FEV1) assessed at 52 weeks. Additional secondary outcomes included ICS dose (defined as budesonide equivalents) and small airway function.
Statistical analysis was performed by the SPSS program for Windows, version 17.0(SPSS, Chicago, Illinois). Data were summarized based on demographic, clinical, and baseline characteristics. Continuous variables are presented as mean ± SD, and categorical variables are presented as absolute numbers and percentages. Normally distributed continuous variables were compared using mean difference and independent sample or paired t-test, whereas the Mann-Whitney U test was used for those variables that were not normally distributed. Categorical variables were analyzed using either the chi-square test or Fisher’s exact test. Paired test or Wilcoxon signed-rank test was used to compare continuous variables from baseline to 16 and 52 weeks, respectively.
A total of 101 patients were included in the study. The baseline characteristics of the patients included in the study are mentioned in Table 1. The mean total serum IgE levels and AEC were 419±303 IU/mL and 300.17±280.11/ cubic millimeter, respectively; further distribution of patients based on IgE and AEC levels is mentioned in Table 1. The mean omalizumab dose/month for patients was 456.46±259.34 mg, with a minimum of 150mg and a maximum of 1200 mg. Results of Skin prick tests and specific IgE testing are mentioned in Table S1 and S2. Most patients (84.2%) reported at least one comorbid allergic condition at study entry.
| Variables | Characteristics |
|---|---|
| Age(y), mean ± SD | 61.13 ± 12.64 |
| Age Distribution(y), n(%) 18 - 30 31 - 40 41 - 50 51 - 60 61 - 70 ≥70 |
2 (2) 4 (4) 19 (18.8) 19 (19.8) 34 (33.7) 23 (22.8) |
| Sex, n(%) Male Female |
40(39.6) 61(60.4) |
| Weight (kg) | 63.64 ± 13.11 |
| The onset of symptoms, n(%) Early-onset asthma Late-onset Asthma(LOA) |
39(38.6%) 62(61.4%) |
| Positive family history of asthma, n(%) |
62(61.4%) |
| Comorbidities, n(%) Diabetes Hypertension Rhinosinusitis (RS) Obstructive sleep apnea Hypothyroidism Osteopenia Osteoporosis Coronary artery disease |
31(30.7) 45(44.6) 80(79.2) 27(26.7) 22(21.8) 18(17.8) 20(19.8) 12 (11.9) |
| Baseline IgE levels n(%) 30 - 250 IU/ml 251 - 700 IU/ml ≥700 IU/ml |
41(40.6) 39(38.6) 21(20.8) |
| Baseline AEC( cells/mm^3), n(%) <150 151 - 299 ≥ 300 |
28 (27.7%) 38 (37.6%) 35 (34.7%)\ |
| Baseline FeNO, n(%) < 20 ppb >20 ppb |
54 (56.8%) 41 (43.2%) |
The number of exacerbations significantly improved (p <0.001) from a mean baseline of 6.34±2.04 to 1.52±0.99 at 16 weeks, 2.81±1.63 at 52 weeks. (Figure 1) There was a statistically significant reduction in exacerbation rates across all groups stratified by baseline AEC, FeNO, and IgE. (Figure S1-S3) Secondary outcomes
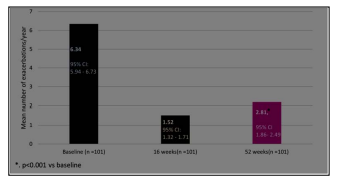
Figure 1: Change from Baseline to Week 52 in annualized rate of asthma exacerbations
We observed a significant improvement(p<0.001) in ACT score, frequency of OCS bursts, and FEV1 at 16 weeks and 52 weeks. (Figure 2a-c)
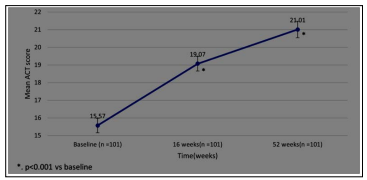
Figure 2a: Change from Baseline to Week 52 in ACT score
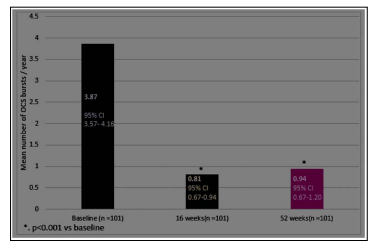
Figure 2b: Change from Baseline to Week 52 in OCS bursts/year
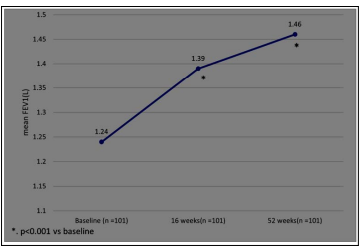
Figure 2c: Change from Baseline to Week 52 in FEV1(L)
There were statistically significant improvements(p<0.001) in hospitalizations/ER visits per year and reductions in mean ICS dosage and mean number of medications prescribed for asthma daily. (Figure S4-S7)
We identified a subset of 23 patients for whom iOS data was available at baseline and 52 weeks. This subset showed no significant improvement in parameters associated with small airway disease (R5%, R20%, and R5-R20), though a significant improvement was seen in FEV1(not shown). (Figure 3a-c) Omalizumab was well tolerated during the study, and no serious adverse effects were reported. The adverse effects are described in Table 2
| Adverse effect | Patients(n) |
|---|---|
| Generalized weakness | 2 |
| Injection site reaction | 2 |
| Gastrointestinal intolerance | 2 |
| Headache | 2 |
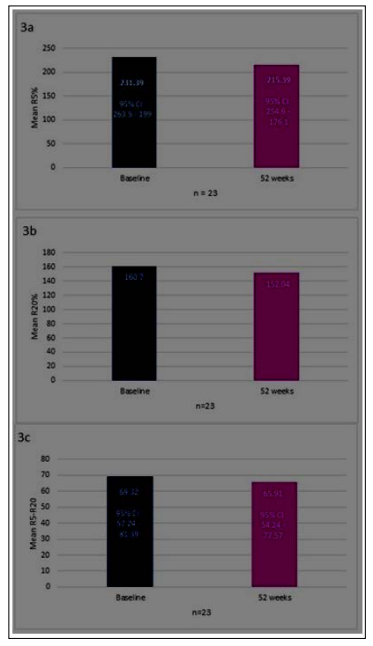
Figure 3: Change from baseline to week 52 in R5%(3a), R20% (3b), and R5-R20(3c)
To the best of our knowledge, this is the first 52-week real-world study that describes the clinical characteristics and outcomes of Indian patients treated with Omalizumab. Despite the limitations (discussed later), our study has several clinically relevant observations. First, omalizumab therapy significantly reduced the annual asthma exacerbations rate of patients in the study. The effect on exacerbations and ACT scores was evident across the groups stratified by biomarkers, i.e., IgE, FeNO, and AEC levels. Inconsistent with previous observations, we did not find an intragroup statistically significant difference in exacerbation rates based on the levels of biomarkers [9]. However, there are reports that pre-treatment biomarker levels might not always predict response [10]. Moreover, the biomarker response has not been extensively studied in the Indian asthmatic population.
Second, the quality of life improved significantly, as demonstrated by an increase in ACT score and a decrease in hospitalizations/ ER visits. We could not calculate the changes in the daily OCS dose, but we did notice a reduction in OCS bursts prescribed to the patient in a year. These results are consistent with other realworld studies on Omalizumab [11, 12].
Third, Omalizumab allowed the reduction of inhaled and oral medications prescribed to patients for asthma. We also observed a reduction in mean daily ICS dose prescribed to patients - this is consistent with previous findings in real-world studies, which demonstrated a reduction in ICS dose by 10-28% after initiation of Omalizumab [13].
Fourth, mean FEV1 increased at 16 and 52 weeks; the absolute mean increase in FEV1 was 190 ml and 220ml respectively, exceeding the MCID for FEV1 (100 ml). There have been conflicting reports regarding the effect of Omalizumab on lung function, but a recent retrospective study in the UK has reported similar improvements in FEV1 [14].
According to this study, the mean age of omalizumab patients was 61.1 years, with only 24.8% patients aged less than 50 years; the mean age of patients in this study is much higher than those reported in other real-world studies on Omalizumab in asthma [15, 16]. LOA might be perceived to be pre-dominantly nonIgE mediated, yet we demonstrated significant improvement in outcomes in this population. [17]. We feel that peculiar social and cultural characteristics in India might have delayed presentation to the clinic. Asthma in the elderly is severe, leading to more impairment of health-related quality of life and higher expenditures vs. asthma in adults(<45 years) [18, 19]. Elderly patients are frequently excluded from randomized controlled trials on asthma because of age and comorbidities. The same has been the case with studies conducted on Omalizumab until now; our research data can help bridge this gap. The data highlight the lack of a set clinical profile for patients who may respond to Omalizumab and suggests that the unmet need is patient-specific.
Severe asthma is frequently associated with comorbidities, with allergic rhinosinusitis being the most commonly reported comorbid condition [20]. The high prevalence of diabetes, hypertension, osteoporosis, and hypothyroidism might be explained by the presence of the elderly population in the analysis. Of interest, it is essential to note the prevalence of OSA, which was present in 26.7% of patients; it has been shown that treatment of OSA with CPAP helps in asthma control [21]. Despite the compelling evidence to evaluate asthma patients for OSA, OSA is largely overlooked in patients with severe asthma in India.
The higher ratio of females to males in the study is explained by a higher prevalence of asthma in adult females vs. adult males [22]. The majority of patients in the study had LOA; this can be explained by a higher prevalence of patients with a family history of asthma and the fact that the presence of a familial history of asthma has been known to confer an increased risk of developing LOA [23].
To date, this is the first time small airway function has been captured in a retrospective study in asthma. It was surprising to note that the small airway parameters(R5, R20, R5-20) did not show significant differences at 52 weeks vs. baseline, despite a significant improvement in FEV1 in this subset of patients. The reasons for this finding are unknown and warrants further investigation.
The safety profile observed in our study is consistent with previous reports [24, 25].
The mean number of exacerbations in the previous year reported in our study are significantly higher than the ones reported in theclinical trials of biologics in asthma. This again might be attributed to a delay in presentation to the tertiary care center, which is quite common in the Indian scenario.
One of the critical limitations of the study is the reliance on clinical records. Incomplete data was seen for FeNO and is possible with OCS bursts/year since patients may self-medicate and fail to report the same. Although the OCS bursts may be underestimated, this is more likely to be in the pre-Omalizumab period since patients will be monitored more stringently after initiation of Omalizumab, thereby underestimating the true effect size of reduction in the OCS bursts after initiation of Omalizumab.
There is a possibility of selection bias since we selected the patients who responded to Omalizumab at 16 weeks.
Except for Omalizumab, the records in the system capture the medication prescribed to the patient. Prescription by HCP does not always guarantee the use of the medication by the patient. However, verbal recall compliance check is routine at all visits at the clinic.
This was a single-center study with a small patient cohort. Consequently, our study may have limited external validity, and the outcomes achieved in the study may not be representative of those at other asthma centers in India. Since the center involved in the current study has a specialized interest in severe asthma, patients are extensively investigated for comorbidities and treated for the same. This may possibly have a bearing on the improvements in QoL, as shown by the ACT, rather than the drug alone.
There is also a possibility of these patients being subsequently classified as “;ACO,”; but with no significant smoking history and /or history of biomass exposure, we feel that the results can be generalized to the asthma population in India.
The lack of control or placebo cohort may mask the spontaneous improvement in asthma or the improvement from frequent contacts with healthcare providers for omalizumab infusion?improved adherence and health-seeking behaviours. However, this is an unlikely confounding factor since most patients were uncontrolled for years before starting on Omalizumab.
However, we need to acknowledge that omalizumab study period is when no other biological was available in our country and may have bearing on selection of biologicals in overlap group which was undefined till more recently
In this real-world study conducted in India, Omalizumab led to improved asthma control, lung function, and QoL and allowed a reduction in the dosage of medications for asthma. The benefit was seen in patients across biomarkers strata and implies that Omalizumab may benefit Indian patients with allergic asthma regardless of biomarker levels and comorbidities. Further studies are needed to evaluate the effect of Omalizumab on small airway function in Indian patients.
What our study adds to existing knowledge
Age of onset of asthma and age when it becomes severe is not the same, and hence, the age of the patient when being evaluated for biologicals need a vigilant appraisal for considering biologicals Biomarkers for severe allergic asthma and demonstrating atopy by SPT or Immunocap assays do not predict response to Omalizumab
Omalizumab in elderly asthmatics with remodeling shows significant and impressive improvements in lung functions besides reductions in exacerbations, OCS use, and hospitalizations.
