Author(s): <p>Nief Rahman Ahmed</p>
A simple, economical and sensitive UV spectrophotometric method has been developed for the determination of allopurinol in environmental wastewater samples and, pharmaceutical preparations which shows maximum absorbance at 250 nm in distilled water. Beer’s law was obeyed in the range of 1 - 20 μg/ ml, with molar absorptive of 0.628 x104 l/mol.cm L.mol-1.cm-1 .The method was successfully applied to the determination of allopurinol in some pharmaceutical formulations (tablets) and industrial wastewater samples. The proposed method was validated by sensitivity and precision which proves suitability for the routine analysis of allopurinol in true samples.
Allopurinol, other name: Zyloric, (Figure .1) is known chemically as 1H-Pyrazolol [3,4] pyrimidin-4-ol.[1].Allopurinol belongs to a group of medicines called enzyme inhibitors.
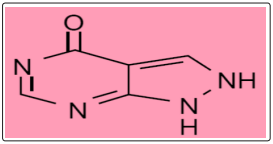
Figure 1: Chemical structure of Allopurinol C5H4N4O
Several methods have been developed for allopurinol analysis, including spectrophotometry,HPLC chromatography techniques , capillary electrophoresis , flow injection technique , Differentialpulse Polarography,and LC-MS/MS method [5-14]. The need in the industry for routine analysis of allopurinol, attempts are being made to develop simple and accurate instrumental methods for quantitative estimation of allopurinol. Thus there is need for the development of new, simple, sensitive and accurate analytical method for the quantitative estimation of allopurinol as an active pharmaceutical ingredient. The present work describes simple and accurate ultraviolet spectrophotometric methods for the estimation of allopurinol in bulk, dosage form and environmental wastewater samples.
Shimadzu UV- 1700 pharmaspec (double beam) spectrophotometer with 1.0 cm quartz cells was used for absorption measurement.
All chemical used were of analytical or pharmaceutical grade and allopurinol standard material was provided from AL-hokamaa company for pharmaceutical industries (HPI) Mosul-Iraq.
Allopurinol stock solution (100 ppm) was prepared by dissolving 0.01g of allopurinol in 100 ml distilled water in avolumetric flask.
The standard solution of allopurinol (5µg/ml ) was scanned in the range of 220-350 nm which shows maxima located at 250 nm Fig 2. Therefore ,250 nm wavelength was selected for the construction of calibration curve.
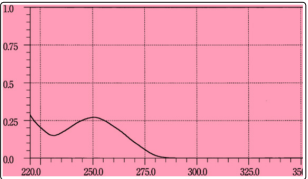
Figure 2: Absorption spectra of (5µg/ml) allopurinol against distilled water
From the absorption maxima ,calibration curve was prepared in the concentration range of 1-20 µg/ml .The absorbance was measured at 250 nm against distilled water as a blank .The concentration of the sample solution can be determined by using the calibration curve.
A real water samples were analyzed by this method. Industrial waste water from AL-hokamaa company for pharmaceutical industries (HPI) Mosul-Iraq , were fortified with the concentrations in the range of 2,10,20 µg/ml of allopurinol. The fortified water samples were analyzed as described above for recommended procedure and the concentration was calculated by using the calibration curve of this method.
Weight and powder 10 tablets. Dissolve a quantity of the powdered tablets containing 0.01 gm of allopurinol in about 100 ml distilled water and mixed for 20 mint and then filtered. The filtrate was mad up to 100 ml with distilled water and aliquot of this solution was treated as described above for recommended procedure and the concentration was calculated by using the calibration curve of this method.
UV Visible spectrophotometry is still considered to be a convenient and low cost method for the determination of pharmaceuticals [15-17]. The method used for the determination of allopurinol in pharmaceutical preparations and environmental wastewater samples was found to be sensitive ,simple ,accurate ,and reproducible . Beer s law was obeyed in the concentration range of 1-20 µg/ml Fig 3 with correlation coefficient of 0.998 ,intercept of 0.0078 and slope of 0.0487 .The conditional molar absorptive was found to be 0.628 x104 l/mol.cm.
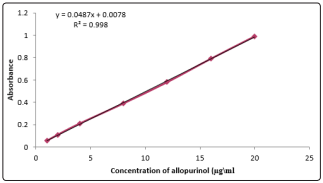
Figure 3: Calibration curve for allopurinol
The accuracy and precision of the method ,a pure drug solution was analyzed at three different concentrations ,each determination being repeated six times. The relative error(%) and relative standard deviation values are summarized in table 1.From table 1 the values of standard deviation were satisfactory and the recovery studies were close to 100%,.The RSD% value is less than 2 indicative of accuracy of the method a Mean of six determinations.
Table 1: Accuracy and precision of the proposed method
| Allopurinol taken ( µg/ml) | Er (%)a | RSD(%) |
|---|---|---|
| 2 | 0.9 | 1.4 |
| 10 | 0.85 | 1.7 |
| 20 | 0.98 | 1.6 |
The proposed method was satisfactorily applied to the determination of allopurinol in its pharmaceutical preparations tablets and wastewater samples ,the results of the assay of the pharmaceutical preparations revels that there is close agreement between the results obtained by the proposed method and the label claim Table 2,and the results of water samples Table 3 show that the recovery values obtained were closed to 100% *Mean of ten determinations.
Table 2: Assay of allopurinol in pharmaceutical formulations
| Pharmaceutical formulation supplied by HPI | Amount of allopurinol * | Label claim | %Recovery |
|---|---|---|---|
| Tablet 10mg | 10.04mg | 10 mg | 100.4 |
| Tablet 30mg | 30.15 | 30 | 100.5 |
Table 3: Determination of allopurinol in spiked industrial wastewater sample
| Water samples | Amount of allopurinol * | %Recovery | |
|---|---|---|---|
| Taken | Found | ||
| Industrial wastewater | 2 | 2.01 | 100.5 |
| 10 | 10.021 | 100.21 | |
| 20 | 20.05 | 100.25 | |
The proposed method proved to be suitable for the content uniformity test, where a great number of assays on individual tablets are required. Data presented in Table 4 indicate that the proposed method can accurately and precisely quantitate allopurinol in its commercially available tablets. The mean percentage (with RSD) of the labeled claim found in ten tablets was (1.8%) which fall within the content uniformity limits specified by the USP 30 [18-21].
Table 4:Content uniformity testing of allopurinol tablets using the proposed method
| Parameter | % of the label claim |
|---|---|
| Tablet NO. 1 | 100. 8 |
| Tablet NO. 2 | 100. 6 |
| Tablet NO. 3 | 99. 8 |
| Tablet NO. 4 | 100.7 |
| Tablet NO. 5 | 99.8 |
| Tablet NO. 6 | 99. 7 |
| Tablet NO. 7 | 99.7 |
| Tablet NO. 8 | 100. 5 |
| Tablet NO. 9 | 100.6 |
| Tablet NO. 10 | 99.7 |
| Mean ( x ) | 100.19 |
| % RSD | 1.8 |
| Max. allowed unit [18] | ±15% |
In this work, a simple, rapid, precise and accurate UVSpectrophotometric method was developed and validated for the determination of allopurinol in pharmaceutical preparations and industrial waste water samples. The method free from such experimental variables as heating or solvent extraction steps. The method rely on the use of simple and cheap chemicals and techniques and can be used for rapid routine determination and quality control of allopurinol in pure form, pharmaceutical preparations and real industrial waste water sample.
The author wishes to express gratitude to the state company of drug industries and medical appliance (HPI) Nineveh - Iraq for providing gift samples of allopurinol standard materials and pharmaceutical preparations (tablets) and for permission and facilities to carry out the research work .
