Author(s): Nkouado Tchando Yannick*, Dulieu Margot, Nankap T Guy and Leach Robert
Background: Chronic nitrous oxide (N2O) poisoning induces irreversible inactivation of vitamin B12, affecting two biochemical reactions essential for the proper functioning of the body. The prognosis is excellent when adequately and quickly treated. We present the case of a 23-year-old Caucasian woman with symptoms caused by chronic N2O inhalation.
Case Presentation: A 23-year-old Caucasian woman was admitted to the emergency department with complaints of confusion and gait disturbance.Following an in-depth history and laboratory tests, chronic N2O poisoning was confirmed, and vitamin B12 supplementation was initiated promptly.
Conclusion: Our case demonstrated the clinical presentation and biological changes typical of N2O poisoning. The misuse of N2O is prevalent due to the easy accessibility of cylinders over-the-counter since a few years. Recreational misuse of N2O has been observed in young populations. It is important to raise awareness among emergency physicians and nursing staff regarding increases in their consumption. Treatment involved vitamin B12 supplementation and long-term addiction monitoring.
Cobalamin, also called vitamin B12, is a water-soluble substance that is not synthesized by the human body but plays an essential role in DNA synthesis and hematopoiesis. It acts as a cofactor in two essential biochemical processes. Nitrous oxide (N2O) is a toxic substance that induces irreversible inactivation of vitamin B12. We decided to publish this case report considering that once a rare pathology, its use is now being widely promoted on social media and there is an increasing trend in the consumption of N2O in the young population of Western countries. It is a medical emergency which presents with characteristic findings on history taking and biological examinations and allows for a rapid diagnosis. Appropriate treatment (vitamin supplementation) will lead to a favorable prognosis.
A 23-year-old Caucasian female patient with confusion and gait disturbances was admitted to the emergency department.
The history provided was not very helpful; the patient's sister-in- law reported finding the patient in a deplorable state of hygiene and making delusional remarks (vision of non-real people) in a depressive context due to romantic separation.
The patient complained of pain in the left calf muscle and felt dyspneic.
She did not have any relevant medical history or take any medication. She had no history of alcohol consumption or smoking addiction.
On admission, blood pressure was 114/62 mmHg, heart rate 100 beats per minute, respiratory rate 22 breaths per minute, oxygen saturation 100% on room air, and core temperature 38.4 °C.
Upon clinical examination, she had poor hygiene, was mentally sluggish and dehydrated. The results of cardiopulmonary and abdominal examinations were normal. In the lower limbs, the patient had pain, redness in the left calf and multiple abrasion- type wounds with signs of erysipelas. Neurological examination revealed confusion and painful paraparesis with hypoesthesia of the left lower limb in the region innervated by L1.
Faced with this clinical picture, we conducted the following additional examinations.
Blood gas analysis revealed pH 7.50 (7.35-7.45), partial pressure of carbon dioxide 25 mmHg (35-45 mmHg), partial pressure of oxygen 128 mmHg (75-90 mmHg), HCO3 19 mmol/L (22.0-29.0 mmol/L), base excess (BE) 2 mmol/L (-2 to 2 mmol/L), SaO2 97.4% (95-99%) and lactate 1 mmol/L (0.50-2.00 mmol/L).
Blood gas analysis revealed pH 7.50 (7.35-7.45), partial pressure of carbon dioxide 25 mmHg (35-45 mmHg), partial pressure of oxygen 128 mmHg (75-90 mmHg), HCO3 19 mmol/L (22.0-29.0 mmol/L), base excess (BE) 2 mmol/L (-2 to 2 mmol/L), SaO2 97.4% (95-99%) and lactate 1 mmol/L (0.50-2.00 mmol/L).
Laboratory tests showed hemoglobin at 7.5 /dL (11.5-15g/ dL), leukopenia at 2.12 10^3/microL (3.8-11.4 10^3/microL) with neutropenia at 0.17 10^3/microL (1.4-7.7 10^3/microL), thrombocytopenia at 133000 (150-455 10^3/microL), disturbed coagulation with Partial thromboplastin time and International normalized ratio outside the normal range, increased D-dimers, C-reactive protein at 316 mg/L (0-5 mg/L), no acute renal failure and no ionic disorders.
Urinary toxicology tests yielded negative results. The entire bacteriological map was sampled.
Electrocardiography revealed sinus tachycardia. Radiological assessment was performed using cerebral and thoraco-abdominal scanners. Right lower lobar and segmental posterobasal pulmonary embolisms in the left lower lobe are highlighted.
Additional laboratory tests and anemia assessment revealed reduced vitamin B12, and thrombophilia assessment revealed markedly increased homocysteine level.
Due to the presence of pancytopenia, pulmonary embolism, and the existing neurological state, we monitored the patient in the intensive care unit.
In severe pancytopenia, an emergency marrow puncture is recommended to rule out acute leukemia.
When the patient was admitted to the intensive care unit, an in- depth history was taken from the patient’s aide (her sister-in-law) who informed us that she had found empty N2O canisters in the apartment.
Given the high suspicion of N2O poisoning, we administered intravenous supplementation with folic acid and vitamin B12. Additionally, for further clarification, levels of methylmalonic acid was tested, which returned positive with a high level of methylmalonic acid.
At the neurological level, cerebral and spinal magnetic resonance imaging (MRI) were performed and returned reassuring. Lumbar puncture showed no abnormalities, and electromyography revealed conduction blocks.
A diagnosis of chronic N2O poisoning was confirmed, and vitamin supplementation was continued. We observed gradual improvement in pancytopenia and encephalopathy.
The patient was then transferred to the neurological rehabilitation department for multidisciplinary care. Despite rehabilitation, the patient required orthoses due to sustained paresis of the elevator muscles of the feet.
Vitamin B12, also called cobalamin, is a water-soluble substance that is not synthesized by the human body but plays an essential role during DNA synthesis and hematopoiesis [1].
It acts as a cofactor in two essential biochemical reactions: in the form of methyl-cobalamin (MeCbl), cofactor of cytoplasmic methionine synthase and in the form of adenosylcobalamin, cofactor of mitochondrial L-methyl-malonyl-CoA mutase [2, 3].
Methionine synthase allows the cytoplasmic formation of methionine and tetrahydrofolate (the reduced and active forms of folic acid) from methyl-tetrahydrofolate and homocysteine. Tetrahydrofolate is then transformed into 5,10-methylenetetrahydrofolate, which is involved in the synthesis of DNA by promoting the methylation of deoxyuridine-5'-monophosphate (dUMP) into deoxythymidine-5'- monophosphate (dTMP). The generation of S-adenosylmethionine from methionine is an integral process in various methylation reactions, including the synthesis of myelin components [4]. Methylmalonyl-CoA mutase allows the mitochondrial generation of succinyl-CoA which enters the Krebs cycle and participates in energy and cellular metabolism. (Fig. 1)
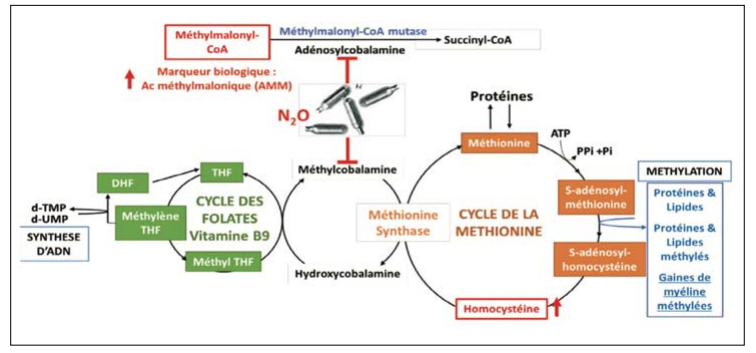
Figure 1: Schematic Representation of Metabolic Pathways Inhibited By Nitrous Oxide
Inhalation of N2O induces irreversible inactivation of vitamin B12 by oxidation of the cobalt atom and blocks the conversion of homocysteine into methionine and the conversion of methyl- malonyl-CoA into succinyl-CoA [5]. This effect appears after 45 min in humans exposed to N20 concentrations above 400 ppm [5]. In general, the clinical signs of vitamin B12 deficiency secondary to a deficiency in intake, absorption, or other conditions develop late, a few years after disease onset (5-10 years). Cobalamin deficiency occurs when hepatic reserves are depleted [6]. This could explain why chronic exposure to N2O was used to identify the symptoms associated with vitamin B12 deficiency. Clinical manifestations may appear early after N2O inhalation in patients with low vitamin B12 reserves [4]. In our case, the patient had recent emotional problems (breakup) and was found with several 2 kg canisters of N2O around her, suggesting a clinical and biological alteration secondary to acute N2O poisoning. Indeed, N2O cylinders are available over-the-counter without any restrictions which promotes access, especially to young people.
The clinical picture of vitamin B12 deficiency secondary to N2O poisoning includes neurological, psychological, and hematological damage.
At the neurological level, inhibition of myelin synthesis leads to demyelination, swelling of axons, and eventually causing loss of substance [7]. The clinical presentation could correspond to demyelinating polyneuropathy, axonal neuropathy or posterior cord syndrome [8, 5]. Electromyography enables the differentiation between demyelinating polyneuropathy and axonal neuropathy. The posterior cord syndrome is characterized by a the so-called "inverted V" T2 hypersignal on magnetic resonance imaging [5].
At the hematological level, frequently observed abnormalities include macrocytosis, anisocytosis, hypersegmentation of neutrophils, non-regenerative macrocytic anemia, thrombocytopenia, leukopenia, and pancytopenia [6, 9].
At the psychiatric level, the mechanism through which N2O inhalation induces psychosis remains unclear. Among the hypotheses mentioned, the appearance of symptomatology would be secondary to cerebral anoxia, methemoglobinemia, and acidosis subsequent to the inhalation of N2O or to the action of N2O on the synthesis of monoamine neurotransmitters, such as dopamine, by increasing the synthesis of BH4 (tetrahydrobiopterin) or due to hypovitaminosis B12 [10]. Clinical manifestations include memory loss, depression, hypomania, dementia, paranoid psychosis with auditory, visual hallucinations and "megaloblastic madness" [7].
Hyperhomocysteinemia resulting from the inactivation of cobalamin represents a risk factor for the occurrence of thromboembolic events [11].
The clinical presentation described above is compatible with that of our patient. If the hypothesis of N2O poisoning had been mentioned upon admission to the emergency room, performing an invasive procedure (marrow puncture or lumbar puncture) or a costly procedure (cerebral MRI) could have been avoided.
The diagnosis of N2O poisoning is often delayed because patients are generally admitted to the emergency room with an altered neuropsychiatric state, which does not allow for reliable history taking, as was the case with our patient. Therefore, it is important to make emergency physicians aware of this condition, its clinical presentation, diagnostic methods, and therapeutic management.
When faced with a clinical picture suggestive of N2O poisoning, the search for vitamin B12 deficiency must be systematic. However, plasma cobalamin levels may be normal because plasma vitamin B12 levels does not differentiate active vitamin B12 and its inactive oxidized form [4]. The markers of choice for all-cause cobalamin deficiency are homocysteine and methylmalonic acid (substrates of reactions catabolized by vitamin B12).
Homocysteine is less specific because it also increases in cases of vitamin B9 and B6 deficiency, kidney failure, rare hereditary metabolic diseases and can be influenced by certain dietary components like alcohol and coffee [4, 12]. Nevertheless, a study carried out by Redonnet-Vernhet et al. on 12 patients admitted to the emergency room of the Bordeaux University Hospital after N2O poisoning showed that only two patients had their vitamin B12 levels below normal, but all presented moderate to severe hyperhomocysteinemia and were recommended systematic measurement of homocysteine suspecting N2O poisoning [13].
The reaction catalyzed by methylmalonyl-CoA is not affected by other vitamins; therefore, methylmalonic acid (MAA) is considered a more specific marker of vitamin B12 deficiency [3]. A plasma increase in the MAA level can be observed in the event of renal failure, intestinal infections or in the event of MMA-CoA- mutase deficiency and is therefore not specific to N2O poisoning [14]. Notably, plasma MAA levels may be normal in the event of chronic N2O poisoning, unlike homocysteine levels, which remains high in patients who take vitamin B12 preventively [14].
In our patient, while assessing for pancytopenia, plasma vitamin B12 level was found to be low, and while assessing for thrombophilia, homocysteine level was found to be high. The methylmalonic acid level was measured later to clarify the diagnosis because vitamin B12 supplementation had already been initiated and was found to be high. Finally, all biological and clinical criteria pointed towards N2O poisoning in the absence of other pathologies.
To date, there are no specific recommendations for the therapeutic management of patients admitted for N2O poisoning. Nevertheless, the complete elimination of this gas is essential. Vitamin B12 supplementation was administered according to the treatment plan recommended for absolute deficiency [5]. Our patient received 1 mg of cyanocobalamin intravenously for ten days, followed by oral maintenance therapy once daily for a total of two months. Partial clinical and biological improvement of the patient were quickly observed. At the end of hospitalization, for one month the patient required neurological rehabilitation for persistent painful paraparesis with hypoesthesia and dysesthesia of the of the left lower limb in the region innervated by L1 .
After follow-up for eight months, we observed persistent paresis of the levator muscles of the foot with slow improvement despite complete removal of N2O, vitamin B12 supplementation, and well-conducted physiotherapy. The patient has not been working since hospitalization. This strongly calls into question the long- term impact of N2O poisoning, both on the health of patients and the socioeconomic level (in an increasingly aging society).
In conclusion, we can state that the problems related to N2O poisoning are secondary to its recreational use among young patients. The availability of this gas over the counter and its addictive nature are the main factors that favor its misuse. It is important to make young people aware of the consequences and long-term effects of acute or chronic N2O poisoning. It is also important to raise the awareness among emergency physicians and nursing staff regarding the clinical presentation and biological changes found in the event of N2O poisoning. This would limit diagnostic delays and reduce the risk of long-term effects of N2O poisoning. This could also help reduce the socioeconomic impact by limiting, the costs incurred for the overall treatment and care of these patients.
1.R Green (2017) Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 129: 2603-2611.
2.M J Oberley, D T Yang (2013) Laboratory testing for cobalamin deficiency in megaloblastic anemia. Am J Hematol 88: 522-526.
3.Luciana Hannibal, Vegard Lysne, Anne-Lise Bjørke- Monsen, Sidney Behringer, Sarah C Grünert U, et al. (2016) Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front Mol Biosci 3 https://www.frontiersin.org/articles/10.3389/fmolb.2016.00027/full.
4.J Blin, M Guerlais, D Masson, A Catteau, S Deheul, et al. (2021) The toxicology of nitrous oxide Nitrous oxide toxicity. Francophone Journal of Laboratories 2021: 48-53.
5.W Caré, L Dufayet, M A Piot, I Crassard, PManceau, et al. (2022) Acute and chronic toxicities associated with the use and misuse of nitrous oxide: review. The Journal of Internal Medicine 43: 170-177.
6.E Andrès, T Vogel, L Federici, J Zimmer, E Ciobanu, et al. (2008) Cobalamin Deficiency in Elderly Patients: A Personal View. Curr Gerontol Geriatr Res 2008.
7.R Sood, T Parent (2022) Peripheral polyneuropathy and acute psychosis from chronic nitrous oxide poisoning: A case report with literature review. Medicine (Baltimore) 101:e28611.
8.S W Kang, J M Hong, D W Namgung, Y C Choi (2019) Neurological Manifestations of Myeloneuropathy in Patients with Nitrous Oxide Intoxication. J Clin Neurol 15: 116-117.
9.G Le Guenno, D Quilliot (2014) Management of cobalamin deficiency in vitamin B12 (cobalamin). Clinical Nutrition and Metabolism 28:130-134.
10.A. Garakani, A. K. Welch, R. J. Jaffe, C. A. Protin, D. M. McDowell (2014) Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics 55: 715-719.
11.M Dematteis (2022) When nitrous oxide no longer makes people laugh: informing users and thinking about it in the face of unexplained neurological disorders. Neurological Practice - FMC 13: 82-85.
12.Netgen (2018) Hypovitaminosis B12: what's new? Swiss Medical Review https://www.revmed.ch/revue-medicale-suisse/2012/revue-medicale-suisse-355/hypovitaminose-b12-quoi-de-neuf.
13.I Redonnet-Vernhet (2022) About 12 cases of nitrous oxide poisoning diagnosed at the Bordeaux University Hospital in 2021: need for measurement of total plasma homocysteine, biomarker of cellular vitamin B12 deficiency. Therapies 77: 785-786.
14.G Grzych, E Gernez, S Deheul, I Kim (2022) Methylmalonic acid: a specific marker of chronic nitrous oxide intoxication? Rev Internal Medicine 43: 197-198.
View PDF