Author(s): Jasmin Ahmad
Objectives: To share the trending of Diabetic Retinopathy (DR) over eight and half years period digital screening program of DR in Bangladesh at a tertiary eye hospital.
Methods: This study analyze 8 and 1/2 years (102 months, from July 2010 to December 2018) findings of a digital screening and subsequent grading programs of DR in a tertiary eye care centre. A total 45289 eyes of 22645 diabetic person underwent retinal photography.7818 (17.26%) retinal Photographs were excluded because of unassessable for grading remaining 37471 retinal photographs were analyzed.
Screening was performed by non-mydriatic digital fundus camera. Optometrist & Ophthalmologist review of all photographs were done at three level of grading system. Evaluation of the percentage distribution of degree of retinopathy was done. Data analysis was done by SPSS software version 16.0.
Results: A total of 22645 patients (64.12 % male,35.88 % female) with mean age of 55.58 ± 9.88 (±SD) years were under went DR screening during a 8.5 years period. Features of DR was found in 18958 of 37471 (50.59%) retinal photographs. Non-proliferative diabetic retinopathy (NPDR) was documented in 14457 of 37471 photographs (38.59 %), while 4501 of 37471 photographs (12.00%) showed proliferative diabetic retinopathy (PDR). Diabetic Macular Edema (DME) was found in 10788 (28.79 %) of total 37471 assessable photograph and (56.90 %) of a total 18958 DR.
Conclusion: DR is highly prevalent among Bangladeshi patients. In order to provide a sensitive, cost effective and easily accessible DR screening, digital imaging is a useful means.
Diabetic Retinopathy is a specific micro vascular complication of diabetes which is the leading cause of blindness among working age group (20 to 64 years) [1].
Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) published epidemiology of DR is the most accepted one [2]. The prevalence of retinopathy is strongly linked to the duration of diabetes. After 20 years of diabetes nearly all patients with type 1 diabetes and over 60% of patients with type 2 diabetes have some degree of retinopathy [2]. The natural history of diabetic retinopathy and the importance of screening must be understood, since even advanced disease can be asymptomatic.
There are currently over 6 million people with diabetes living in Bangladesh. The number is expected to dramatically increase to more than 11 million by 2030 making Bangladesh the seventh largest number of diabetes in the world [3].
Screening for diabetic retinopathy saves vision at a relatively low cost than the disability payments provided to blind person in the absence of a screening program [1].
Diabetic Retinopathy was defined according to the International Clinical Diabetic Retinopathy Severity Scale adopted by American Academy of Ophthalmology (AAO) and the International Council of Ophthalmology (IC O).
The project was approved by the institutional review board and ethical approval for this study was granted by the Institute of Community Ophthalmology.
It is a cross sectional study and purposeful sampling method was applied.
Both eyes of each participant were photographed by technicians with a 45-degree digital non-mydriatic camera (Canon, Lake Success, New York, USA). For each eye two photographic fields were taken; the first centered on the optic disk (field 1), and the second centered on the fovea (field 2). Retinal photographs were evaluated by graders at 3 level of grading (primary by health technician, secondary by optometrist & evaluated finally by ophthalmologist).
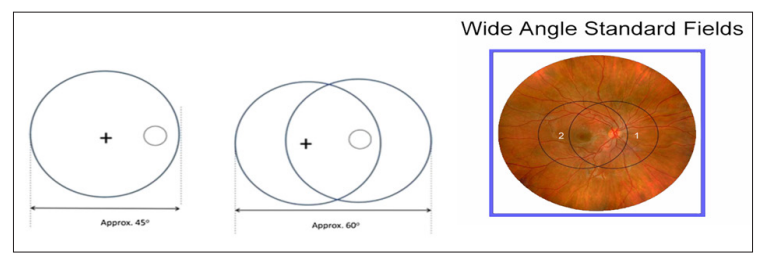
Figure 1: Wide angle 45-degree standard field retinal photography, two photos cover approximately sixty degrees [4].
The grading procedures explained in this protocol are a modification of the ETDRS5 protocol and the NSC(National screening commete, UK) Proposed Grading criteria6, Retinal photography were graded into no diabetic retinopathy at level R0, mild-moderate NPDR( microaneurysm(s), retinal haemorrhage(s) ± any hard exudate ) at level R1 and severe-very severe NPDR(venous beading, venous loop or reduplication, intraretinal microvascular abnormality (IRMA), multiple deep, round or blot haemorrhages ) at level R2 & proliferative retinopathy PDR (new vessels on disc (NVD), new vessels elsewhere (NVE), pre-retinal or vitreous haemorrhage, pre-retinal fibrosis ± tractional retinal detachment) at level R3 [5, 6]. (Table :1)
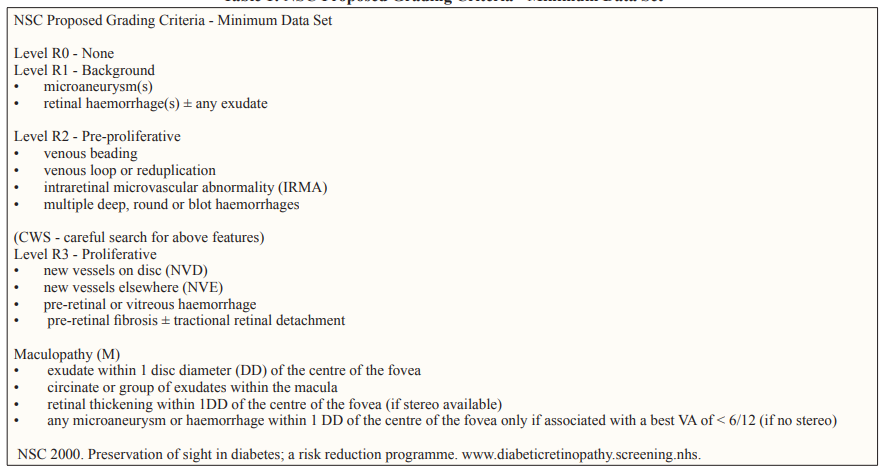
Clinically significant macular edema (CSME) was considered to be present when exudate within 1 disc diameter (DD) of the centre of the fovea, circinate or group of exudates within the macula, retinal thickening within 1DD of the centre of the fovea (if stereo available), any microaneurysm or haemorrhage within 1 DD of the centre of the fovea only if associated with a best VA of < 6/12 (if no stereo) [6]. (Table: 1)
For this study we excluded all the unassessable/ungrabable photographs.
Informed consent was obtained from all diabetic person underwent screening during the study period.
A total of 45289 eyes of 22645 diabetic persons under went retinal photography during a 8.5 years period of screening. Among them 14519 (64.12 %) were male and 8126 (35.88 %) were female. The mean age of the participants was 55.58 ± 9.88 (±SD) years with range, 14 to 100 years.
7818 (17.26 %) retinal photographs were excluded because of unassessable for grading.The remaining 37471 retinal photographs were analyzed. (Table-2). The cause behind poor quality of image was not mentioned in the data.
18958 (50.59%) of 37471 assessable photographs showed features of different grades of DR. (Table-2)| Year | Number ofPhotograph | Unassessable | Assessable | >Number of DR | Percentage of DR ( %) |
|---|---|---|---|---|---|
| 2010 | 1344 | 56 | 1288 | 664 | 51.55 |
| 2011 | 4293 | 75 | 4218 | 1416 | 33.57 |
| 2012 | 4350 | 109 | 4241 | 2404 | 56.68 |
| 2013 | 4354 | 149 | 4205 | 2133 | 50.73 |
| 2014 | 3882 | 275 | 3607 | 1499 | 41.56 |
| 2015 | 5392 | 787 | 4605 | 1949 | 42.32 |
| 2016 | 6584 | 1851 | 4733 | 2689 | 56.81 |
| 2017 | 6892 | 2003 | 4889 | 3273 | 66.95 |
| 2018 | 8198 | 2513 | 5685 | 2931 | 51.56 |
| Grand Total | 45289 | 7818 | 37471 | 18958 | 50.59 |
| Grading of DR | Number | % |
|---|---|---|
| R1 | 11407 | 60.2 |
| R2 | 3050 | 16.1 |
| R3 | 4501 | 23.7 |
| Total number of DR | 18958 | 100 |
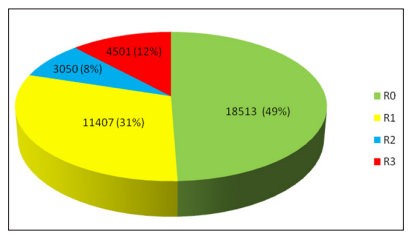
Figure 2: Rate of Retinopathy grading (2010-2018)
A total of 18513 of 37471 images (49.41 %) were graded R0, means no features of diabetic retinopathy (Fig:2) 11407 photographs (60.2 % of DR and 30.44 % of total assessable photograph) showed mild to moderate NPDR (R1); 3050 photographs (16.1 % of DR and 08.14 % of total assessable photograph) showed severe to very severe NPDR (R2) and the features of PDR (R3) found in 4501 photographs (23.7 % of DR and 12.0 % of total assessable photograph). (Table -3) (Figure-2)
10788 photographs (56.90 % of 18958 DR & 28.79 % of 37471 assessable photographs) showed features of DME. (Fig-3)
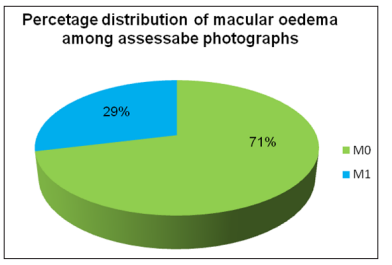
Figure 3: Percentage distribution of macular oedema (2010- 2018)
During the study period of screening, the different grades of retinopathy findings over the years shown in figure 4 and figure 5 shows the distribution of macular oedema with DR through out the years.
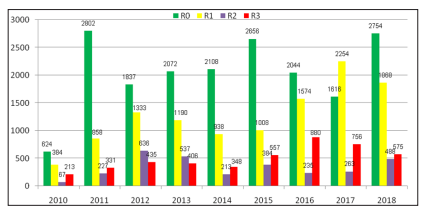
Figure 4: DR Grading distribution over the years of screening
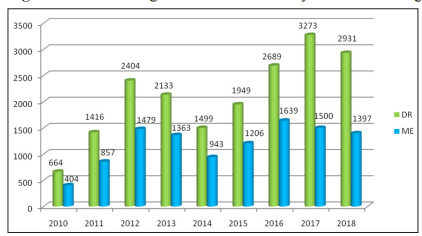
Figure 5: Distribution of DME with DR over the years of screening
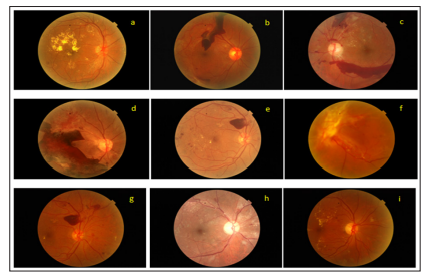
Figure 6: Example of Macula- centered and Disc -centred screening retinal photographs with good vision (6/6 to 6/12) - a. NPDR with DME (6/9) b. PDR with vitreous haemorrhage (6/6) c. PDR with pre retinal haemorrhage (6/9) d. PDR with pre retinal haemorrhage with TRD(6/12) e. PDR with pre retinal haemorrhage with macular oedema(6/9) f. PDR with vitreous haemorrhage with TRD (6/12) g. PDR with pre retinal haemorrhage with macular oedema (6/9) h. PDR with venous chage with macular oedema (6/9) i. NPDR with DME (6/6)
Diabetic retinopathy fulfills all the four-cardinal principle of disease screening by WHO those are, 1)The condition should be an important health problem with a recognizable pre symptomatic state 2) [7]. An appropriate screening procedure which is acceptable both to the public and health care professionals should be available.3). Treatment for patients with recognizable disease should be safe, effective and universally agreed.4). The economic cost of early diagnosis and treatment should be considered in relation to total expenditure on health care, including the consequences for leaving the disease untreated.
Screening for DR is very important as it is remain silent at a very advance stage of disease (fig: 6).
Many different modalities of screening for DR are in use depending on local availability of facilities.The method which is used should have sufficient sensitivity (>80%) and specificity (>80%).
In the developing countries there are not a sufficient number of ophthalmologists to undertake annual retinal examination for all diabetics. Screening provided by general practitioners often appears to be inadequate.
Previously the implicit "gold standard" for identifying and grading retinopathy is a seven field stereoscopic photographs of each eye (This needs two frames from each field to simulate a stereoscopic view; thus, fourteen frames from each eye are needed). interpreted by experienced readers. Recording and archiving of retinal images were traditionally been done using 35-mm slides or Polaroid prints [8]. The photographs can be taken by a technician and are later assessed by a trained reader or an ophthalmologist. This method is well suited to serve communities [9-11]. Single-field 45-degree images of the disc centre and macula centre are found to be highly correlated (k = 0.97, P = 0.0001) to the gold standard of the stereoscopic seven-field mydriatic images [12-14]. More over two single field photographs reduce the cost, complexity and the time spent, storage and reproduction are inexpensive.
We found an overall 18958 of 37471 assessable photographs
(50.59%) showed features of DR, and 10788 of 37471 assessable
photographs0 (28.79%), (56.90 % of 18958 DR) showed CSME
So more than half of retinopathy had features of macular oedema
which was associate with poor vision.
To our knowledge this study provides the first eight and half
year’s data extracted from a digital screening program of diabetic
retinopathy in Bangladesh. In Bangladesh there are very few data
on diabetic retinopathy prevalence.
Muquit and and his associates found (7 years screening result)
over all prevalence of DR in Bangladesh across 3 centre was 33%
while they found 64.6% at CEITC ,39.8% at NIO and only 13.0%
at a diabetic hospital [15].
According to Kazi Rumana, Bangladesh Diabetic Shomity, there
are at least 1.5million people with DR. 0.75m with severe DR,
Incidence rate of DR (95%CI) 23.54, 17.52, 21.47 per 1000-person
year at 5,10,15 years diabetic age [16]. Hazrat Ali et al found
5.2% of total population were suffering from diabetes,among
them 26.2% people suffering from retinopathy [17]. In our study
the prevalance of DR is (50.59 %) was quite higher and nearly
similar to the 7 years findings of Muquit and his co-worker as it
was done in a tertiary eye hospital and also there may be some
extant of hospital biasness [15].
Rajiv Raman and his associate found the prevalence of diabetic
retinopathy in the Indian urban population with diabetes mellitus
was 18.0% [18]. Sunil Gupta et al showed 19 in their study that
34 % of type 2 diabetics had DR. Tien Yin Wong et al found an
overall prevalence of retinopathy of 33.2%, CSME of 5.6%, among
participants with diabetes [19, 20]. We found 28,79 % CSME in
total assessable photograph.
Out of 37471 assessable photography 18958 (50.59 %) were detected with different grades of DR. Among them we found the mild to moderate NPDR-11407/37471 [30.45%], severe to very severe NPDR-3050/37471[08.14 %], proliferative DR (PDR)- 4501/ 37471 [12.0%]. CSME was seen in 10788 out of 18958 case of DR (56.90 %). Muquit et al found the prevalence of PDR 16.05 % at CEITC,11.5% at NIO and 1.3% at a diabetic hospital [15]. Tien Yin Wong et al found the prevalence as per the severity of DR was mild NPDR-97/350 [27.7%], moderate to severe NPDR-21/350[6.0%], proliferative DR (PDR)- 1/350 [0.3%] [20]. Tien Yin Wong et al also found CSME in 21 out of 119 case of DR (17.9%) [20].
The higher percentage of sight threatening DR (PDR, DME) in our study is probably due to lack of awareness and ignorance about visual health among population as well as poor improvement in the clinical management of diabetes and nation-wide less health coverage in this developing country like Bangladesh.
Other studies by Amos AF et al found the 34% prevalence of
diabetic retinopathy, DR prevalence found by Harris MI et al,
Australian Diabetes Study, and Blue Mountains Eye study showed
the prevalence as18.2%, 15.3% and 35.5% respectively [21-24].
The strength of this study is that it used digital fundus photography
and the high frequency of gradeable fundus photographs and the
use of standard grading protocols by trained grader.
Limitations of this is its hospital biasness. The study does not evaluate the risk factors like duration of diabetes, blood sugar, lipid profile, blood pressure, smoking. And also, does not evaluate the visual status and treatment.
Diabetic retinopathy screening is now a time demanding issue. Therefore a sensitive, cost effective and easily accessible screening method should be provided, funded and audited. It provides early detection and treatment of DR and hence reduce the load of irreversible blindness in the society.
Financial Disclosure: None reported.
Support: Dr. Ahmadur Rahman Research Centre
