Author(s): Lucia K Kiio*, Damaris N Mbui, Peter M Ndangili, Onyatta J Onam and Florence Oloo
Cancer, the abnormal and uncontrolled cell growth due to an accumulation of specific genetic and epigenetic defects, has posed a particular threat to human health which makes it to be ranked third in Kenya as a cause of death after infectious and cardiovascular diseases. The unregulated cell growth leads to the formation of a tumor, which after some period of time spreads beyond the site of origin and metastasizes to other body organs and systems, making it incurable. Early detection of cancer (long before the tumor is perceived) greatly increases the chance of curability, and is the point of focus for many cancer researchers. Lung cancer is the primary cancer threatening human life worldwide and its mortality rate has not decreased for a number of years. The mortality rate can be reduced by early diagnosis and subsequent treatment, for example if the serum proteins, microRNA, tumor-associated antigens, are detected in elevated amounts in the blood of potential lung cancer patients using a highly sensitive biosensor. Throughout the years, researchers worldwide have extensively investigated many screening modalities for lung cancer detection, including chest radiograph (CRG), computed tomography (CT), low-dose CT (LDCT), magnetic resonance imaging (MRI), and positron emission tomography (PET) and biopsy. However, these techniques are not suitable for patients with other pathologies. Developing a rapid and sensitive technique for early diagnosis of lung cancer plays an important role in early diagnosis and subsequent treatment. The typical methodologies limit the applications due to the low selectivity, low sensitivity, high cost, low stability and invasive procedure. Tumor markers as biochemical parameters can reveal cancer occurrence and progression, which show selectivity, stability sensitivity, convenience, and low cost in developing biosensors, and act as good prospect for fabricating biosensors of detecting lung cancer. This review portrays several biosensors (2010-2019) for detection of lung cancer biomarkers. To start with, cancer in general and Lung cancer has been briefly described focusing on the screening methods that have been used for lung cancer. The currently used diagnostic tools for lung cancer with their advantages and disadvantages is also portrayed. overview of the application of transducers used, including optical, mass-based, electrochemical and calorimetric biosensors, in lung cancer detection, together with the advantages and disadvantages of the development of new biosensors. Finally, the recent challenges and further opportunities for developing effective biosensors for early diagnosis of lung cancer are discussed.
Cancer diagnosis and treatment is of great concern due to the widespread occurrence of cancer cases, high death rate, and recurrence after treatment. According to Melonie et al; 2006 in the National Vital Statistics Reports, the rate of incidence (per 100,000 persons) of cancer in white people was 470.6, in black people 493.6, in Asians 311.1, and Hispanics 350.6, indicating that cancer is wide- spread among all races. Lung cancer, breast cancer and prostate cancer were the three leading causes of death in the US, claiming over 227,900 lives in 2007 alone [1,2].
According to global cancer statistics reports, Lung cancer is the leading cause of cancer related death worldwide [3]. In Kenya, cancer ranks third as a cause of death after infectious and cardiovascular disease [4]. It causes 7% of total national mortality every year. Although population-based data does not exist in the country, it is estimated that the annual incidence of cancer is about 28,000 cases and the annual mortality is over 22,000. Over 60% of those affected are below the age of 70 years. In Kenya, the risk of getting cancer before the age of 75 years is 14% while the risk of dying of cancer is estimated at 12% [4]. According to the National Cancer Institute, cancer is greatly feared due to recurrence. Although tumors can be treated, they can return after a period of time, even after chemotherapy, surgery, or radiotherapy. Cancer onset and progression is accompanied by mutated or aberrantly expressed proteins which would evoke immune response, resulting in the production of autoantibodies. These antibodies in cancer patients could be detected months or years before the clinical diagnosis of cancer. Survival of a cancer patient depends heavily on early detection and thus development of sensors for detecting cancer at an early stage would be especially useful in the war against cancer.
Existing cancer screening methods include the Papanicolau test for women to detect cervical cancer, mammography to detect breast cancer, prostate-specific antigen (PSA) level detection to detect prostate cancer, occult blood detection for colon cancer, endoscopy, Lobectomy for lung cancer CT scans, X-ray, ultrasound imaging and Magnetic Resonance Imaging (MRI). These diagnostic methods however are not very useful for early cancer detection [5]. Additionally, some of the screening methods are quite costly and not available for many people. Therefore, the development of technology that is specific, reliable and easily accessible for early detection of cancer is of utter importance.
Lung cancer is a major health problem worldwide. It is the most commonly occurring cancer in men and the third most commonly occurring cancer in women. There were 2 million new cases in 2018 [6]. Previous studies have indicated that tobacco smoke environmental pollution, second-hand smoke industrial substances and genetic factors may cause lung cancer. Compared to some other common cancers such as breast cancer, lung cancer continues to have a much lower survival rate. Early diagnosis of lung cancer with suitable treatment significantly improves the fiveyear survival rate. Chemotherapy and radiation therapies are commonly applied for small cell lung carcinoma (SCLS), while surgical treatments are normally provided for non-small cell lung carcinoma (NSCLS) [7-13].
Some lung cancer screening methods, for example chest radiograph (CRG), computed tomography (CT), low-dose CT (LDCT), magnetic resonance imaging (MRI), and positron emission tomography (PET) have been studied extensively. These techniques have some drawbacks, such as being expensive and having low sensitivity for identifying cancer cells at early stages. Annual CRG was reported as not helpful in reducing the mortality of lung cancer. CT has been considered as the gold standard lung cancer screening tool, which offers information on tumor features such as size, characterization and tumor growth. 3D CT image offered assessment of the chest wall, diaphragm, and mediastinum invasion, in addition to staging the tumor. Radiations produced from CT also increased the cancer risk. To solve this limitation, LDCT was applied for lung imaging and it reduced 20% of lung cancer mortality. However, LDCT continues to have a high false positive rate (up to 96.4%). 18F-Fluorodeoxyglucose PET/CT was applied in oncological imaging but produced inaccurate results. Magnetic induction tomography (MIT) has been recently proposed for early disease detection with the advantage of high-sensitivity but disadvantage of high cost [14-20].
Apart from imaging approaches, biopsy is another common way to identify lung cancer; however, it is expensive and requires trained physicians [21].The major limitations of the existing diagnostic methods include being time-consuming, expensive, and having low-sensitivity for low cancer cells concentrations [22]. Therefore, it is necessary to develop a rapid, low-cost and high-sensitive method for early diagnosis of lung cancer. Biomarker-based techniques for early diagnosis of tumor markers have attracted much attention [23]. Autoantibodies can detect lung cancer cells about five years earlier than autoradiography because tumor growth is associated with gene and protein changes [24].
Such methylation or point mutation of DNA, RNA and mutated or aberrantly expressed proteins, carbohydrates, cytokines and chemokines, as well as volatile organic compounds from the peroxidation of the cell membrane species could be detected months or years prior to clinical diagnosis and are able to act as cancer biomarkers. According to, tumor-associated autoantibodies for NSCLC could be detected 5 years before it could be detected using autoradiography [21, 25].
The most frequent method to test serum Tumor associated antigen (TAAs) is ELISA and solution hybridization detection method for miRNA. To detect TAAs, monoclonal antibodies and aptamers are usually used as capture agents while for miRNA, capture agents used are usually the corresponding single- stranded DNA (ssDNA). These methods are time consuming, expensive and is not sufficiently sensitive for the low marker concentrations at early cancer stages [26].
Fabrication of a Nano-structured electro-analytical biosensor for early detection of lung cancer could effectively reduce mortality rate due to lung cancer.
| Diagnostic method | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Biopsy | Fast and easy | Inflammation, painful, invasive | [27] |
| Sputum cytology | easy and noninvasive | Degradation of biomarkers due to enzymes in sputum, false positive results | [28] |
| Chest X-ray | Quite reliable | Use of radiation, false negative response, high cost | [29] |
| Magnetic resonance imaging | Quite reliable | Use of magnetic field, high cost, not suitable for all patients that have other complications | [30,31] |
| Computed tomography | Quite reliable | High cost, false negative scans, use of radiation | [32] |
| Positron emission tomography | Quite reliable | Need for radioactive substance and sophisticated instrument, not suitable for all patients who have other complications, high cost | [33] |
Currently, lung cancer is detected mostly in the late stages exhibited by symptoms such as coughing, including coughing up blood, shortness of breath, and chest pains. The early stages of this disease are often detected ?by accident?. Chest radiography and computer tomography are the most commonly used methods for lung cancer diagnosis [34]. However, these can only identify visible and irreversible changes in the lung. In order to enable and facilitate cure of lung cancer, there is for early diagnosis, which can be achieved, for example by highly sensitive and specific biomarkers.
In Kenya, the Nairobi cancer registry (2013) in a report, reported that 34 people out of 100,000 have been afflicted by lung cancer, with a male to female ratio of 2:1. Locally, this malignancy is the seventh and tenth most common for males and females respectively. Worldwide, it is the second most common cause of mortality amongst cancer sufferers [35]. The predisposing factors include: smoking (both active and passive which is responsible for up to 80 - 90% of cases), asbestos exposure, family history of lung cancer, chronic lung diseases, prior history of lung cancer and air pollution.
A biosensor is a bio analytical device incorporating a molecular recognition entity associated with or integrated with a physicochemical transducer [36]. When constructing a biosensor, three components should be considered, a bio-recognition element for selective recognition of an analyte also known as bio receptor, an immobilization matrix for the immobilization of a recognition biomolecule and a transducer for conversion of biochemical response into a measurable signal [37]. Bio-receptor and transducer together are referred to as a biosensing membrane.
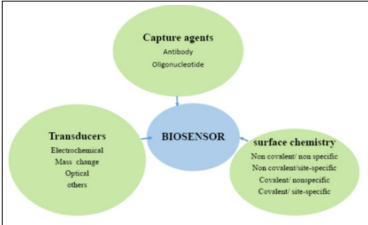
Figure 1: The three components of biosensors [37].
To detect cancer antigens, monoclonal antibodies and aptamers are often used as capture agents. They capture micro Ribonucleic acids (miRNAs) corresponding to single stranded Deoxyribonucleic acid (ssDNA). A transducer is a device that converts the molecular recognition signal to an electrical signal. The transducer may be electrochemical (by Potentiometry, amperometry, conductometry / impedimetry), optical (fluorescence, luminescence, colorimetric and interferometry), calorimetric (thermistor) or based on mass changes (piezoelectric / acoustic waves.
Biosensors have a number of potential advantages over other methods of cancer diagnosis such as, reduced assay time, portability, high sensitivity and selectivity, simplicity, miniaturized and flexibility. Biosensor-based diagnostics can assist cancer screening and improve the rates of early diagnosis and attendant improved forecast. This technology can be particularly useful for enhanced healthcare delivery in the public setting and to underserved Diasporas. Biosensors have potential for multi-target analyses, automation, and cost-effective testing [38].
Broadly, biosensors are categorized according to their transduction or Biological element. These biological elements are generally enzymes, micro-organisms, antibodies, and biological tissue immobilized on a transducer surface. These transducers are enlisted mainly in optical, calorimetric, electrochemical, or mass based, depending on the development. For intercommunication between biorecognition elements and a specific target, a biomarker is enforced to produce signals that can be amplified. Other very important facets of the biosensor are; detection limit, linear range response time, and immobilization techniques, depending on the type of transducer used [39].
The types of biosensors can be summarized using figure 2.
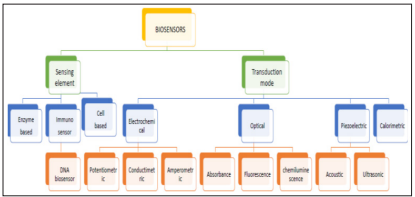
Figure 2: Types of biosensors [40].
Among the various biosensors, electrochemical biosensors have received special attention as they allow high sensitivity, lower detection limits, automation, inexpensive testing, and development of disposable devices and methodologies capable of working with very small sample volumes.
The electrochemical biosensors have also shown a colossal potential to furnish a high sensitivity, higher selectivity and lower limit of detection (LOD) in the diagnosis of lung cancer as well as detecting multiple markers concurrently [41,42]. Biosensors can also be classified according to the type of recognition material applied. First, a distinction can be made between enzymatic or bio catalytic and affinity biosensors. An enzyme biosensor is derived from a combination of a transducer with a thin enzymatic layer, which normally measures the concentration of a substrate [43]. The enzymatic reaction transforms the substrate into a reaction product that is detectable by the electrode. The concentration of any substance can be measured provided that its presence affects the rate of an enzymatic reaction which is especially true for enzyme inhibitors. The signal that is current or potential measured is proportional to the rate-limiting step in the overall reaction [44]. Bio catalytic sensors use enzymes, cells or tissues as bio receptors. In affinity-based biosensors antibodies, receptor proteins, nucleic acids or bio mimetics are utilized as recognition element [45,46]. Affinity biosensors can be further differentiated into direct or indirect sensors.
The classification, shown in Figure 2, depends on whether or not the presence of a labeled element is needed for the generation of the sensor signal. The preference goes to label-free or direct biosensors because they make real-time measurements possible, they are less expensive and there is no need for extensive sample preparation. In labeled or indirect biosensor formats, the signal is not generated by the analyte receptor complex itself, but by a secondary element, the label bound to the complex. This kind of biosensor is derived from immunoassays for example, sandwich complexes [26, 46-49].
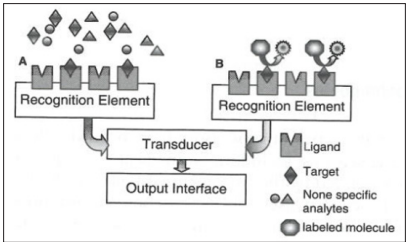
Figure 3: Schematic representation if (A) a direct biosensor and (B) an indirect biosensor using a sandwich assay [48].
| TRANSDUCER | SAMPLE | LOD | LINEAR RANGE | REFERENCE |
|---|---|---|---|---|
| SPR | Serum | 50 nM | - | [50] |
| SPR | Blood | 1.35 x 10-4 nM | 3.64 x 10-4-3.64 x 102 nM | [51] |
| Fluorescence | Serum | - | 1.25 pM-1.25 μM | [52] |
| Piezoelectric (SPR and QCM) |
Serum | - | 0.03-2 μM | [53] |
| Impedimetric | Serum | 10-300 pM | 1 pM | [54] |
| Electrochemical | Serum | 0.05 nM | - | [55] |
| Electrochemical immunosensor |
Serum | - | 5 nM-50 nM | [56] |
| Amperometric | Saliva | 0.167 nM | 0.5-500 nM | [57] |
| Amperometric | Serum | 10 pM | 1.25 pM-1.25 μM | [58] |
| Impedimetric | Serum | 0.06 nM | 0.01-1000 nM | - |
| Optical (FRET) | Serum | 1.7 pM | 1.7 pM | [59] |
| Electrochemical | Serum | 1.7 pM | 4-100 pM | [60] |
| Electrochemical immunosensor | Serum | 0.05 nM | 0.1-2000 nM | [61] |
The molecular recognition element must be able to detect low concentrations of the target molecule in a mixed population, so there is a need for high specificity and sensitivity. To address these requirements, antibodies are often used. According to Bohunicky and Mousa, 2011, antigen- and antibody-based recognition elements are not only very specific, which leads to low detection limits, but the detection system is also very fast. Both polyclonal and monoclonal antibodies have proved to be successful. However, monoclonal antibodies tend to be more specific. Polyclonal antibodies are generally cheaper, but show varying affinities. Extensive research is being done to replace the antibodies by synthetic recognition elements, such as peptides, aptamers, nanomaterials and molecular imprinted polymers. This will increase the stability and reproducibility of the biosensor [26,46,62-64].
An electrochemical biosensor is an analytical device that is fabricated by modifying the surface of an electrode with biomolecules, such as enzymes, antibodies, and DNA [65]. This biosensor is based on electrochemical techniques in which analyte sensing is made by measuring the electrical response as an analyte reacts electrochemically with the surface of the working electrode of the sensor [66]. Ideally, such a device is capable of responding continuously and reversibly and does not perturb the sample. Electrochemical biosensors combine the analytical power of electrochemical techniques and the specificity of biological recognition processes. The most commonly used transducers in electrochemical biosensors are Amperometric and potentiometric transducers. The analytical information in potentiometric devices is obtained by converting the biological response or biochemical reaction into a potential signal by the use of ion selective electrodes.
Amperometric biosensors on the other hand monitor the current generated against applied constant potential by the reduction or oxidation of the electro active species involved in the bio recognition process [67]. The enhanced sensitivity, specificity, simplicity, and inherent miniaturization of modern electrochemical bioassays allow them to compete with the most advanced optical protocols [68].
Different optical-based biosensors have been developed for early diagnosis of lung cancer markers and the techniques have been enhanced by administering nano-techniques and surface chemistry [69]. The optical biosensor is an appliance that measures changes in the optical properties of substances, from absorbance in chemical reaction, reflectance, refractive index, fluorescence, phase shift, and light energy (wavelength) [70]. The device is the most prevalent type of biosensor and is extensively used in surface plasmon resonance (SPR), evanescentwave fluorescence, and bioluminescent optical fiber biosensors in conjunction with ellipsometric and reflectometric interference spectroscopy, interferometric, and surface-enhanced Raman scattering biosensors [71].
Currently, most commercial platforms use fluorescence detection systems, while most research tools use grating coupler and resonant optical biosensors [72]. SPR-based biosensors have been developed for biomolecular interaction SPR-based sensors excite surface plasmon from the interface and measure the refractive index changes, which can be classified as label-free and real-time affinity reaction detection systems.
The application of a biosensor in the diagnosis of lung cancer has drawn the attention of researchers. SPR-based biosensors have been developed for biomolecular interaction [72]. SPR-based sensors excite surface plasmon from the interface and measure the refractive index changes, which can be classified as label-free and real-time affinity reaction detection systems. The SPR biosensor responds to refractive index adjustment connecting the sensor surface, combining with the bind of the particular substance; for example, a cancer biomarker with an enzyme adhered to the sensor surface will generate changes in reflectivity [73]. The optical-based biosensor has been utilized for lung cancer biomarkers as an excellent, technology-driven diagnostic tool. A study has showed the SPR-based biosensor for the detection of the cancer biomarker carcinoembryonic antigen (CEA) with a high sensitivity and in optimized condition emanated to high sensitivity and reproducibility for CEA detection. The advanced immunosensor applying the sandwich assay format has verified to be successful, and is an auspicious tool for analysis [74].
The application of the optical biosensor has both pros and cons: the pros are that it is small, flexible, and safe, no electrical device connects with the body, and it has good biocompatibility because the fibers are made of glass [70]. The cons are that it may be invasive, and the fluorescence may not be strong enough.
Research is being carried out on a possible new mediation to test for tuberculosis (TB), by coughing into the tube of the breathalyzer and bringing up sputum, leading to a positive reading if TB is present in the lung Figure 4 shows the SPR detection principle [70].
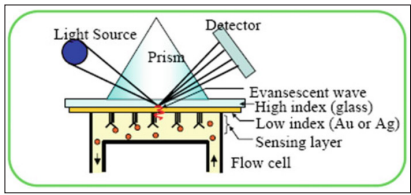
Figure 4: The SPR detection principle schematic [71].
There are two types of biosensors that rely on mass changes that is Acoustic and piezoelectric biosensors. Piezoelectric biosensors are crucial for bioanalytical applications with numerous advantages which include; easy-to-make, high-sensitivity and cost-effective. Piezoelectric quartz crystal (PQC), a thin slice of quartz obtained from a single crystal with optimal chemical, electrical and mechanical properties, is pertinent for analytical applications.
Quartz crystal microbalance (QCM) -based sensors have been enforced for point mutation detection of lung cancer Piezoelectric biosensors are based on the principle of acoustics (sound vibrations) [75]. A quartz crystal microbalance (QCM) is one such sensor that measures the alteration in frequency of a quartz crystal resonator as a mass per unit area. Essentially, the working principle of the piezoelectric biosensor is that an electrical signal is produced when a mechanical force is applied. The sensing molecules adhere to the surface, and a mass to frequency transducer results to mechanical vibrations from the interaction between the analyte and the sensing molecules, transforming it into an electrical signal corresponding to the amount of the constituent. The use of piezoelectric biosensors in cancer detection has proven useful by immunosensors and microcantilever biosensors. Most immunosensor applications have involved the human p53 gene. The coupling established the principle’s ability to recognize point mutations in that particular gene (the most common in most types of cancer) with PCR amplification [76].
Commonly, different piezoelectric biosensors have been developed depending on the variant biomolecules, comprised of antibodies, nucleic acids, and receptor, which attach to the sensor in the medical application site [77]. For improved lung cancer diagnosis, a novel piezoelectric biosensor employing a lead titanate zirconate (PZT) ceramic resonator as transducer granted cost-effective, label-free, and direct disclosure of cancer biomarkers resulting in high sensitivity (0.25 ng/ml) and rapid diagnosis (within 30 min) utilizing the prostate-specific antigen (PSA) in the performance evaluation [78].
There is nonetheless a limitation on the biosensor. While the piezoelectric biosensor is available in the desired shape and size, and has a better frequency response, it also leads to hightemperature sensitivity and is not suitable for measurement in a static condition in which it reacts more towards the dynamic [79].
A calorimetric biosensor usually measures the heat absorbed or evolved during a chemical reaction or change of state. The working principle mainly illustrates the measurement of exothermic reactions. The enzymatic reactions generate the heat: thus, fluctuations in temperature will be executed to determine the concentration of the analyte [80]. Variation results in the enthalpy drives subsequent to the monitoring process. The biosensor is not widely and principally implemented in cancer diagnosis, but has still been improved as a tool for clarification and analysis. For instance, in the use of a calorimetric sensor coupled to aptamerbased gold nanoparticles, two different types of cells, acute leukemia and Burkitt’s, have been successfully distinguished [81].
Recently, an advanced calorimetric biosensor was created for the detection of cancer biomarkers by Au Nanoparticles Bi2Se3 Nanosheets. The improved system was based on the high catalytic activity of the biomarker by merely connecting an Au precursor with the as-synthesized Bi2Se3 nanosheets in an aqueous solution, resulting in fluctuation in color, contributing to the high sensitivity and selectivity of the cancer biomarker even at a concentration as low as 160 pg/ml for the carcinoembryonic antibody (CEA) [82].
The drawback with calorimetric biosensor is first, their relatively low sensitivity which limits their applications in clinic detection. Additionally, there is difficulty in assuring that the temperature of the sample stream is consistent, but the major concerns are its applicability and provision of strongly colored solutions.
In conclusion, it is clear that lung cancer has the highest mortality rates of all cancers due to its short survival period. Biosensor technology is the most reliable method and is likely to provide alternate tools for early lung cancer detection and hence a reduction in death rates. There is a likelihood, therefore, that an advanced biosensor will soon be utilized in therapeutic treatment for cancer diagnosis. The biosensors continue to evolve especially electrochemical biosensor with improved selectivity and sensitivity to develop new analytical strategies and applications for biomarkers determination. However, they still have a long way ahead before becoming a competitive technology for development of point-of-care diagnostic testers [83,84].
Most common approaches are prepared from platforms of a single biomarker design. However, only few devices have detected three or more markers, and they still exhibit the challenge of selectivity. The development of new distinguishable species involving nanomaterials and electroactive probes which can produce more than two independent electrochemical signals, is a major area of concern. The target will be to develop a non-invasive, portable, and cost-effective biosensor which also be highly-specific, sensitive, versatile, and reliable.
However, it is worth to mention also that despite the tremendous progress that has been made in these last years in the development and application on ultrasensitive immunosensing platforms for multiplexed determination of clinical biomarkers, none has crossed the technological valley of death to successful commercialization. Recurrently, the validation of the electrochemical immunosensors developed is limited to doped biological samples such as blood, serum, saliva and urine or to a very limited number of real samples, which is not enough to ensure their reliable validation.
Despite these formidable challenges to address the attractive properties of these electrochemical biosensor, electrochemical immunosensing devices make them extremely promising for improving the reliability and agility of diagnostics and therapy monitoring, leading to more rapid clinical decision making and corresponding reductions in patient stress and healthcare costs. This will assure them a privileged place in the future clinical field.
Acknowledgments: Financial support of the Gandhi Samrak Nidhi fund is gratefully acknowledged.
Conflicts of Interest: The authors declare no conflict of interest.
