Author(s): Lena Marinova*, Vaska Vassileva, Viktor Petrov, Iliya Gabrovski and Galin Vulchev
Anaplastic meningiomas are rare aggressive brain neoplasms that are followed by recurrences, despite a gross total surgery.
We present a 45-year-old woman with an anaplastic meningioma recurrence two years after surgery, which is not followed by postoperative raditherapy. Through this clinical case, we emphasize the required diagnostics and complex treatment in analaplastic meningiomas as well as the current optimization options of imposing surgical intervention, radiotherapy and drug treatment.
?he standard care is maximum safe resection followed by adjuvant raditherapy (RT) for grade 3 meningiomas. Radiosurgery (RS) is an effective therapeutic alternative for intracranial and spinal meningiomas up to 3.5 cm. Hypofractionated radiosurgery (HFRS) is administered postoperatively in analaplastic meningiomas with a diameter of more than 3.5 cm. After re-operation of recurrences and after 6 months of the previous radiotherapy, it is possible to conduct HFRS re-irradiation, followed by targeted therapy.
The meningiomas are extraaxial brain tumors originating in the upper layer of meningo-endothelial arachnoid cells. Supratentorial intracranial meningiomas are most commonly diagnosed [1]. Although most meningiomas are benign (80%) and slow-growing, atypical (15-20%) and anaplastic (1-3%) meningiomas are more aggressive with a tendency to recurrence, worse clinical outcomes, and higher disease-specific mortality [2-6]. WHO 2016 definition of anaplastic meningiomas is a meningioma that exhibits overtly malignant cytology (resembling that of carcinoma, melanoma or high grade sarcoma) or markedly elevated mitotic activity [7]. Through the clinical case with an anaplastic meningioma recurrence two years after surgery, we emphasize the required diagnostics and complex treatment in analaplastic meningiomas as well as the current optimization options of imposing surgical intervention, radiotherapy and drug treatment.
We present a 45-year-old woman with a relapse of anaplastic G3 meningioma. On the occasion of a stubborn headache in November 2017, imaging studies and brain MRT were conducted, which establish a right temporal brain tumor (Figure 1). On December 7, 2017, visibly total tumor resection and pathohistologically verified as anaplastic meningioma / G3 was performed. Intraoperative description - Subdural found a core of a greyish and licked brain tumor as well as a large cyst on the right reaching the lateral ventricle. Immunohistochemical analysis - Ki67-3 + (> 30%) - high proliferative activity; 100% expression to Vimentin; CK AE1 / AE3 - missing expression. The patient is not targeted for postoperative radiotherapy. On Control CT / April 2018- The famous cystic zone was visualized to a right temporally pulling the rear edge of the right lateral ventricle, that wass taken as a post-operative cyst. During the beginning of 2020, the headache recovered MRT / May 2020. establishes recurrent tumor mass data in the operative bed (Figure.2) and from MRT /October 2020, the recurrent lesion is increased by 32.5 mm/18mm (Figure.3). On 28.10.20 re-surgery of tumor relapse was performed. Postoperative intensity modulated radiotherapy (IMRT) using the VMAT in the volume of the tumor bed with 2 cm insurance zone with daily dose (DD) 2 Gy up to total dose (TD) 58 Gy (Figure.4) was performed. After 1 year of radiotherapy, the patient is without relapse, with good quality of life and without neurological symptoms.
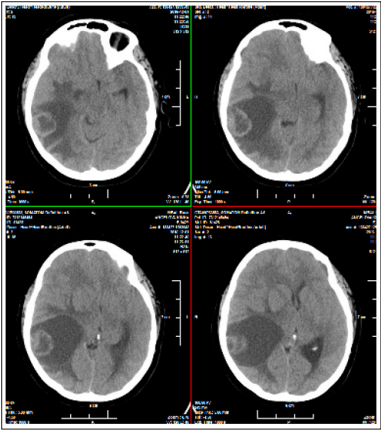
Figure 1: Brain CT / November 2017 of a brain tumor on the right temporal with expressed perifocal edema and the dislocation of the medium brain line
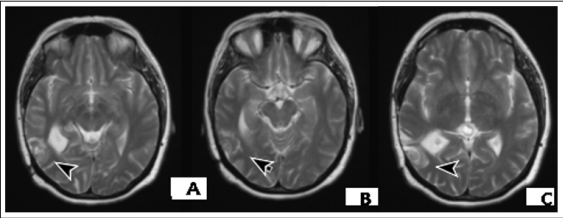
Figure 2: Brain MRI / May 2020- Recurrent temporal tumor in the volume of the previous surgery via December 2017

Figure 3: Brain MRI / October 2020 - A/ Increased brain lesion with a diameter of 32.5 mm / 18mm in comparisons with MR image in May 2020, with irregular tumor borders, heterogenous tumoral enhancement and perifocal vasogenic edema; B/ Postoperative pelvis MRI / March /2012- Residual sacral chordoma with progression in size and expansion to the right gluteal muscles (27).
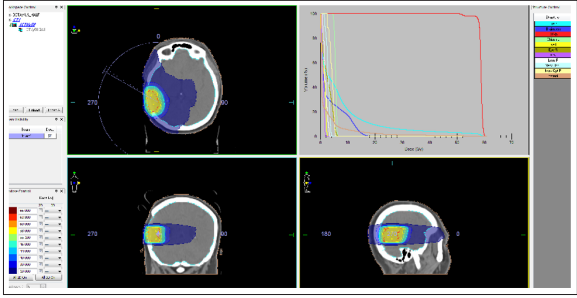
Figure 4: Intensity modulated radiotherapy (IMRT) using the VMAT in the volume of the tumor bed with 2 cm insurance zone with daily dose 2 Gy up to total dose 58 Gy.
Meningiomas are characterized by a diverse pathohistological characteristic and different mitotic activity. Benign G1 meningeomas are diagnosed in 90% such as meningo-endothelial, fibrous, transient, psamomatous, angioblastic/ mostly aggressive; Atypical G2 meningeomas in 7% - choroidal, clear cell, atypical / infiltrating brain; Anaplastic malignant G3 meningeomas in 2% - papillary, rabdoid, analaplastic [1, 8]. The clear cell and chordoid subtypes are also considered atypical [3, 4, 9]. In 2007, the WHO criteria were further modified to remove the automatic classification of tumors with brain invasion into the grade 3 category, thereby further increasing the proportion of grade 2 to the range 20%-35% [10]. Grade III (anaplastic) meningiomas exhibit histologic features of overt malignancy, including high mitotic activity (20 or more mitotic figures per 10 high power microscope fields), frank anaplasia with focal, or diffuse loss of meningothelial differentiation, and their cytology often resembles carcinoma, sarcoma, or melanoma [11]. Atypical and anaplastic meningiomas occur more frequently over the cerebral convexities than at the skull base. When these high grade meningiomas occur at the skull base, they have lower recurrence rates and better overall prognosis than similar tumors found over the convexities [12, 13]. High levels of progesterone receptors are associated with favorable prognosis, whereas meningiomas with loss or absence of progesterone receptors tend to be more aggressive with increased rates of recurrence [14-18]. Distant metastases from meningiomas are extremely rare, occurring in an estimated 0.1% of meningiomas and almost always in association with very large intracranial tumours [19]. Tumor cells may pass the bloodbrain barrier and metastasize into the lung, liver and bones [1]. The overall and disease-free survival are directly dependent on the mitotic cell activity of G2 and G3-meningeoms, respectively 11.9 years / 3.3 years and 11,5 years / 2.7 years [8]. For higher grade tumours, in the US National Cancer Database (1985-1992), five year overall survival rates of 75% for atypical (grade 2) and 55% for malignant (grade 3) meningiomas were recorded [20].
Difficult Differential Diagnosis (DD) in the like pathochistological characteristics of cranial extraosal Ewing sarcoma / pPNET, carcinomeningioma, analaplastic G3-meningioma, atypical G2-meningioma and hemangiopericitoma requires immunohistochemical (IHC) analysis [8]. The phenotypical malignant transformation of meningial tumor cells from classic atypical meningioma is characterized by intermediate filament protein, prominent by increased expression of GFAP, vitamin, alpha-internexin, neurofilament protein (NFP), desmin and keratin [21]. P53 is believed to have a significant role in the pathogenesis of meningosarcoma [22]. Intermediate fylamen protein is a normal finding during central embryogenesis. The malignant progression of the meningioma cells reduces the expression of EMA and cell metaplasia increases alpha-internexin and NFR. IHC-analyzes determine claudin-1 for a specific marker in atypical meningioma [23]. IHC in the analaplastic G3 meningioma / meningosarcoma reports a positive membrane reaction to CD34, Ki 67- 3+ (> 30%) - high proliferative activity, 100% expression to Vimentin; negative expression to CK AE1 / AE3 [7]. In addition to the aforementioned WHO criteria, the mouse intestinal bacteria 1 (MIB-1) proliferation index is a histopathological biomarker that is associated with higher recurrence rates in meningiomas [24]. In the presented clinical case, IHC analysis reports Ki67 > 30%- high proliferative activity; 100% expression to Vimentin and negative expression of CK AE1 / AE3. The lack of expression of CK AE1 / AE3 sharply distinguishes this lesion from carcinoma neoplasms. Interesting is DD of chordoid subtypes meningiomas, which diagnose <1% of all meningiomas, and chordomas that are axially located brain tumors. These meningiomas are called “chordoid” because it resembles a chordoma, with cords or trabeculae of epithelioid cells that may be eosinophilic or clear. Chordomas are characterized by positive IHC expression to Cytokeratin and EMA, while chordoid subtypes meningeomas are negative to Cytokeratin but positive to EMA [25]. MRI features that may be associated with higher grade meningiomas include markedly irregular tumour margins, irregular nodules, a mushrooming pattern and inhomogeneous enhancement [26]. In the presented clinical case MR image of relapse, namely irregular tumor borders, heterogenous tumoral enhancement and peritumoral edema (Figure.2, Figure.3/A), resembles the MR image of chordoma (Figure.3/B) [27].
Treatment of Intracranial Meningiomas is imposed in progressive or rapidly explained neurological symptoms. Surgical treatment includes a radical operation along with the infiltrated soft brain shell and bone [28-30]. Gross total resection (GTR) is the preferred primary treatment, and this can be further classified into Simpson grades 1-3 (G1-G3) resection, where G1 resection removes tumor, underlying bone, and dura, G2 resection removes tumor and coagulates the attached dura, and G3 resection removes tumor but leaves the dura intact [31]. The standard of care is maximum safe resection followed by adjuvant radiation for grade III and incompletely resected grade II meningiomas [11]. Practice shows a direct relationship between the volume of surgery and the risk of relapse [29]. GTR plus adjuvant radiation for malignant meningiomas has been shown to independently predict improved disease-free and overall survival times - five-year disease-free survival improved from 15% without radiation to 80% with adjuvant radiation [32]. After a macroscopic radical resection, including the brain shell with the infiltrated bone, the level of recurrences reached 10% after coagulation up to 20%, and after partial resection - up to 74% [4, 5, 33, 34]. The meningiomas localized in the sphenoidal area, as well as parasagittal are most commonly recurrent, due to gross surgical removal difficulties. The rare meningiomas of the cavernous sinus, tentorium and cerebellopontine angle are extremely difficult for radical operation due to postoperative severe neurological and sensory deficits [2]. Following complete resection, the 5-year recurrence rate is 29-58% for atypical and 72-94% for anaplastic meningiomas [5, 35, 36]. Literature published since the WHO 2000 classification report higher recurrence rates at five years following surgical excision for WHO grade II (41%) and III (70-91%) than for WHO grade I lesions (3%) [37-39]. Non-skull base location, male gender, and prior surgery are all independent pre-operative risk factors for WHO grade II or III meningiomas. This increased risk translates to probable poorer prognosis and increased likelihood of recurrence after treatment [16]. Patients with subtotal resection/ Simpson grade IV resection, who received RT had fewer recurrences and higher PFS compared to those who did not. 3-yr OS was equivalent in both G2 and G3 tumors with and without RT. G3 histology and Simpson grade were predictive for relapse while brain invasion, mitoses, adjuvant RT, and location of tumor were not [31]. The Simpson grade of meningioma resection was described in 1957 and correlated the degree of surgical resection completeness with symptomatic recurrence [40]. Risk of recurrence was similar after grade I and II resections, tended to increase after grade III (HR: 2.35; p = .087) but was higher after grade IV resections (HR: 7.35; p = .003) [41].
Radiotherapy (RT) is an effective and generally well-tolerated treatment for meningiomas. Based on evidence in the literature, adjuvant radiation is usually recommended for atypical meningiomas following incomplete resection, for anaplastic meningiomas regardless of the extent of resection, and for recurrent meningiomas [42-44]. Post-operative RT is standard due to a high level of recurrence at G3-meningiomas [45]. In the presented clinical case, an anaplastic meningioma was diagnosed after 2.5 years of diagnostics and first surgery. The patient has not conducted postoperative RT, which is an omission in the complex treatment and the main reason for the relapse (Figure.2, Figure.3/A. Maire JP et al., Report a good radiotherapeutic effect after partial tumor resection in relapses, inoperable meningiomas and analaplastic, aggressively growing tumors, despite the maximum radical surgery [46]. A more pronounced radiosensibility of partially resected meningiomas compared to recurrences was observed [28, 47]. RT is the treatment choice when meningiomas are considered as unresectable either due to tumour location (most commonly in close proximity to the optic apparatus or in the skull base) or when the patient requires treatment but is not suitable for surgery [48]. In the past 10 years, the role of radiosurgery (RS) in the meningiomas was reported [49-53]. RS delivers a single high dose is generally used in atypical and anaplastic meningiomas with residual or recurrent disease. Treatment doses typically range from 12 to 20 Gy [54-56]. Displayed for RS are meningiomas with dimensions up to 3.5 cm, localized to 5 mm from the optic nerve or chiasm, with good accumulation of CT or MRT contrast [57]. Stereotaxic RS is administered to patients with surgery contraindications with comorbidity or elderly [51, 58, 59]. After the realization of RS with high single fractions, there were no late radical effects involving dementia or secondary radioinduced tumors [60]. Risk factors associated with developing perilesional edema ranging from 2.5 to 50% include prior radiation treatment, larger tumor volume, higher tumor grade, and parasagittal location [61, 62]. In meningiomas with a larger volume and a diameter of more than 3.5 cm, it is possible to conduct a hypophractioned RS by which high radiation doses of 15-35 Gy over 3-6 fractions [63, 64]. Subsequently, fractionated stereotaxic RT for inoperable meningiomas or for patients with comorbidity was introduced [65]. A phase II trial of IMRT administered radiation doses ranging from 54 to 60 Gy in 30 fractions for treatment of incompletely resected atypical meningiomas, anaplastic meningiomas regardless of extent of resection, and recurrent meningiomas [11]. Pirzkall et al. compared conformal and IMRT plans used in nine patients and showed that the IMRT technique increased dose and target coverage while sparing OAR [66]. Re-irradiation with photons is challenging due to the surrounding healthy tissue’s limited tolerance to more radiation; however, particle therapy has been described as safe and effective for re-irradiation in recurrent or progressive meningiomas [67]. Due to the large volume of post-operative tumor bed, in the recurrent anaplastic mengioma, we conducted the IMRT using the VMAT method with a 1cm insurance area with DD 2 Gy up to TD 58 Gy (Figure.4). The patient suffered well, without early radiation effects. After 1 year of RT, the patient is without relapse, with good quality of life and without neurological symptoms. Despite surgery and RT, in recurrent and aggressively growing meningiomas, hydroxyurea has been imposed [1, 68]. Several studies using bevacizumab, a monoclonal antibody against the VEGF receptor, have reported mild improvement in PFS in patients with recurrent meningiomas [69-71]. In phase II clinical trials of sunitinib for recurrent and progressive atypical and anaplastic meningiomas, there was a PFS of 42% at 6 months, which was an improvement from reported natural history PFS of 5-30% at 6 months [72].
Anaplastic meningiomas are diagnosed in 1% to 3% of all meningioma lesions. Their differential diagnosis requires a strict pathohistological study with immunohistochemical analysis. MR image of relapse, namely irregular tumor borders, heterogenous tumoral enhancement and peritumoral edema, resembles the MR image of chordoma. The standard care is maximum safe resection followed by adjuvant RT for grade 3 meningiomas. Radiosurgery is an effective therapeutic alternative for intracranial and spinal meningiomas up to 3.5 cm. Hypofractionated radiosurgery is administered postoperatively in analaplastic menangiomas with a diameter of more than 3.5 cm. After re-operation of recurrences and after 6 months of the previous radiotherapy, it is possible to conduct HFRS re-irradiation, followed by targeted therapy
