Author(s): Hatim abid*, Mohammed EL Idrissi, Abdelhalim EL Ibrahimi, Abdelmajid Elmrini, Nissrine Amraoui, Sara Elloudi, Hanane Baybay, Fatima Zahra Mernissi
We report in the light of a literature review the results of 18 patients followed for stage III cutaneous melanoma in lower limb who underwent a primary melanoma resection, complete inguinal lymph node dissection extented when necessary, to iliac and obturator nodes.
Of all malignancies, cutaneous melanoma has the most rapid increase in incidence over the recent decades [1]. It constitutes 1.6% of all estimated new cancer cases worldwide (42,084 cases in 2012, excluding non-melanoma skin cancer), and the burden is similar for men (1.5%) and women (1.6%). Globally, melanoma ranks as the 15th most common cancer in women and 16th most common in men. [2,3].
Surgical therapy remains the primary and most effective intervention for this disease [1]. Lymph node metastasis is a powerful predictor of recurrence and death in patients with cutaneous melanoma [4]. Complete lymph node dissection (CLND) is a cornerstone in the treatment of patients with cutaneous malignant melanoma (MM) with a positive sentinel node biopsy (SNB) [5]. We share through this article our experience concerning the management of inguinal node metastatic disease for stage III cutaneous melanoma by CLND as part of a multidisciplinary approach.
Eighteen patients (10 women, 8 men) underwent complete inguinal lymph node dissection for the melanoma of lower extremity between January 2011 and December 2016 were included in this study. All patients were histologically diagnosed and classified according to the American Joint Committee on Cancer (AJCC) TNM system prior to surgery. We included only patients classified stage III. The main clinical characteristics of the 18 patients included in the study are summarized in the Table 1. Node metastatic disease was diagnosed with the use of ultrasound (US) and computed tomography (CT).
Surgery was performed according to a standard procedure in patients with clinical or radiographic groin disease. Inguinal lymph node dissection was performed through a standard 12 cm incision extending from 2 cm below the inguinal ligament to the apex of the femoral triangle. All the fibrofatty tissue, extending from the external oblique aponeurosis 2 cm above the inguinal ligament to the medial border of the adductor longus muscle medially and sartorius muscle laterally, was removed. At the end of completion of the dissection, suction drains were used routinely. All the patients were administered with low molecular weight heparin 6 hours postoperatively for deep vein thrombosis prophylaxis and prophylactic antibiotics. Postoperatively all patients were called for regular visits during which prospective assessment of the wound complications as well as clinical locoregional inguinal lymph nodes recurrences was performed. In addition, ultrasound of the lymph node basin and liver as well as chest x-ray are made if necessary.
The median age at diagnosis of the melanoma was 54 years (range 39 - 68 years). The average Breslow depth of the primary melanomas was 4,2 mm (1,2 mm - 8 mm). Five patients underwent inguinal dissection after groin lymphadenopathy was noted on physical examination at the time of primary lower extremity melanoma diagnosis. 3 patients had wide spread in-transit metastasis, and the other 10 patients underwent dissection for primary thick melanomas (> 4 mm). The mean follow-up period was 36 months. Four patients presented local recurrences and only one patient who had widespread in-transit metastasis at the first admission developed pulmonary metastasis 6 months after the surgery. Five short-term complications (28%) were observed: Seroma and hematoma formation were noted respectively in 3 and 2 patients without occurrence of wound infection or dehiscence. Short-term lymphedema formation was observed in 3 patients around the 6th week without persisting at the last follow-up.
Melanoma is the cancer with the most rapid increase in incidence. The highest incidence rates occur in populations of white Europeanancestry residing in geographic areas with high UV exposure. The age-standardized rate (ASR) is 35.1/100,000 for Australia/ New Zealand compared with 13.8/100,000 for North America, 14.6/100,000 for Northern Europe, 12.1/100,000 for Western Europe, 8.1/100,000 for Southern Europe, 1.6/100,000 for South America, 1.1/100,000 for Africa, and 0.5/ 100,000 for Asia. Rates are generally higher in men compared with women and in older compared with younger age groups [3]. In our context, it is still impossible to determine the incidence of melanoma at the national level due to the absence of a national cancer registry.
In the early twentieth century, and only at the basis of clinical impressions of surgeons, the recommended treatment of primary melanoma was aggressive surgery, involving wide resection margins of 3 - 5 cm around the primary melanoma and, frequently, elective radical dissection of the regional node [6,7,8]. Many studies have subsequently showed that the extent and aggressiveness of regional surgical therapy did not have a significant impact on melanoma specific survival [9,10].
Indeed, the results of several randomized and nonrandomized studies provide the basis for the current recommendations about the width of surgical margins, based on primary melanoma thickness also known as the Breslow measurement. For melanoma in situ, there have been no randomized trials and only a limited number of case series to guide excision margins which are 5 mm suggested by the European guidelines [11,12] and 10 mm announced in the British Best Practice statement [13] particularly in the case of lentigo maligna. For a thickness of less than 2 mm or more than 2 mm, 1 cm and 2 cm margins respectively appear to be adequate [14,15,16]. In this context, no data exist that margins wider than 2 cm result in any superior disease-specific outcome [17]. In our practice we take into account the Breslow measurement to determine excision margins which varied between 1 and 2 cm.
For regional lymph node dissection for melanoma, attitudes are divided between complete lymph node dissection (CLND) and sentinel lymph node biopsy (SLNB). The CLND is indicated in the cases of patients with clinically evident nodal metastases identified radiographically or by physical examination [18]. In this context, the nodal involvement at the time of diagnosis is considered as the only predictor of outcome [9,10]. For the SLNB, a recent American Society of Clinical Oncology (ASCO) and Society of Surgical Oncology (SSO) clinical practice guideline reviewed multiple studies and suggested that SLNB should be considered in melanoma whose thickness is less than 0.8 mm with high risk features and for thickness between 0.8 to 4.0 mm [19].
Concerning the CLND, studies have suggested on the one hand that extensive lymph node dissection has a significant impact on both disease free and melanoma specific survival and the other hand have indicated that reducing the extent of surgery increases the risk of local failure and leads to lower survival rates. Previous studies have also suggested that CLND improves local and regional disease control particularly when the procedure is performed in a melanoma treatment center [18,20]. In addition surgical dissection plays a major role in the staging of ilio-obturator nodal disease and prevents the morbidity of pelvic dissemination, which is associated with poor quality of life [21]. However this technique is the subject of a main criticism represented by a hight rate of complications such as Infection, hemorrhage, skin-flap necrosis, wound dehiscence, paresthesia and lymphocele with chronic lymphedema [22].
At the lower limb, in an effort to minimize short- and longterm morbidity associated with the classic longitudinal “lazy-S” incision, several modifications have been proposed concerning first the modifications to the length and position of the traditional incision [23]. For this, alternatives are represented by shorter vertical incisions that cross the groin crease at right angles, transverse incision parallel to the groin creas separate and incisions oriented along lines of skin tension above or below the groin crease. The other modifications relate preserving the adductor magnus, the sartorius fascia, the saphenous vein and transposition of the sartorius muscle to cover the femoral vessels [23].
For improving the substantial morbidity associated with open CLND, Bishoff et al [24] first described in 2003 the successful use of an endoscopic approach to groin dissection in cadaveric specimens with penile squamous cell carcinoma. Then the technique was modified and adapted to the inguinal lymphadenectomy performed in patients with melanoma which allowed complications related to inguinal incision [25,26,27]. In a fairly small cohort of patients (N=25) who have undergone the procedure, the complication rate was 12% (3 of 25 dissections) which is significantly lower than 50% published in recent series of open groin dissection [1]. Solari et al had a significant reduction of postoperative wound complication rate that was around 4%. A multicenter study involving a group of melanoma surgeons from high volume United States centers showed that the videoscopic inguinal lymphadenectomy can safely and adequately be performed after a structured training program with a relatively short learning curve [28,29]. The lymph node count is an important surrogate marker for the quality of the lymph node dissection whatever the technique adopted. According to literature, the recommended number of harvested lymph nodes for the inguinal lymphadenectomy in melanoma patients varies from 5 to 10 [30,31]. In our context, we practice the open CLND procedure with great conviction in the cases of patients with clinically evident nodal metastases identified radiographically or by physical examination staged III according to the American Joint Committee on Cancer (AJCC) TNM system.
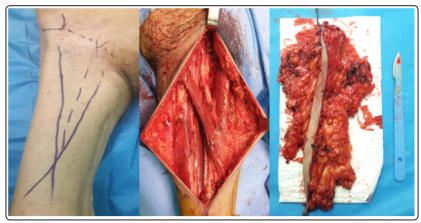
Figure 1: Intra-operative aspect of complete inguinal lymph node dissection
Combined iliac-obturator to inguino-femoral lymph node dissection is clearly indicated in the setting of biopsy-proven disease without distant metastases [23]. Other studies have established relative indications to associate iliac-obturator node dissection including Radiographic suspicion of pelvic disease, involvement of three or more inguinal nodes, extracapsular tumor extension of inguinal nodes, a large (>3 cm) positive inguino-femoral node, in the case of a positive Cloquet’s node whose sensitivity and specificity are highly variable and in patients who have recurrent melanoma of the extremity and have already undergone an inguinofemoral lymphadenectomy [23,32-35] Access to the iliac fossa is gained via a transverse incision through the external oblique aponeurosis approximately 5-6 cm above the inguinal ligament. This procedure can be practiced using the robotic-assistance that has been shownto improve visualization of the iliac and obturator nodes, provides equivalent nodal yield and shorter length of stay when compared to the classic technique [36]. In our series we practiced iliac lymph node dissection in association to inguinal lymphadenectomy in 6 patients which represented 33% of the series.
Concerning popliteal lymph node in patients with melanoma, its incidence is exceedingly rare. In one study examining 4262 patients seen at the Sydney Melanoma Unit during a 30-year period, the incidence of clinical involvement of popliteal nodes was determined to be 0.3% [36]. Thereby, Thompson et al recommends popliteal lymph node dissection only for clinical evidence of metastatic disease in a popliteal node. The surgery is performed on a patient in the prone position by a lazy-S incision made over the flexor crease to allow optimal exposure and heal in a manner that does not cause a joint contracture [37,38].
Regarding the sentinel lymph node biopsy (SLNB), Morton and colleagues had introduced its concept for melanoma in 1992 and established the validity of the procedure through the Multicenter Selective Lymphadenectomy Trial-1 (MSLT-1) published in 2006 [39]. This technique which is justified by the results of several studies such as those of Veronesi et al and Balch et al who estimate that patients are subjected in the case of CLND to unnecessary surgery nearly 85% of the time [40,41]. Sentinel lymph node biopsy is based on the well-supported premise that lymphatic metastases from melanoma follow an orderly progression through afferent lymphatic channels to sentinel lymph nodes before spreading to nonsentinel lymph nodes and subsequent distant sites [42].
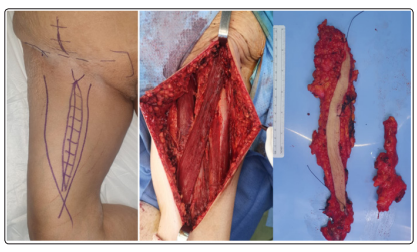
Figure 2: Intra-operative aspect of complete ilioinguinal lymph node dissection
Breslow depth is a direct predictor of the risk of nodal metastases. The likelihood of detecting positive nodes on SLNB is approximately 1.7% for lesions of 0.76 mm in thickne, 3.9% if the lesion is between 0.76 and 1.0 mm, 7% for lesions between 1.0 and 4.0 mm thick and more than 20% for lesions of 4.0 mm [43,44]. SLNB is now considered to be the standard of care in those patients with primary melanomas of 1.0 mm in thickness with clinically negative nodal. Many melanoma experts currently use a threshold of 0.76 mm in depth as an indication for SLNB especially in the presence of high-risk characteristics (ulceration, mitotic rate 0.1/mm2, lymphovascular invasion, and those with ambiguous depth secondary to a positive margins) [45,46,47]. In addition to these histologic risk factors, age has been shown to be a factor in the risk of sentinel node involvement. Indeed younger patients having a higher incidence of nodal metastases than older ones [47]. In our contexte, we do not practice the SLNB because we are faced the barrier of limited access to the patented blue dye, radioisotope tracers, isosulfan blue, and nuclear medicine facilities. In addition we consider the high false negative rate of this procedure announced by the American Society of Clinical Oncology and which ranged from 4.6% to 16.7% [48,49].
In the meta-analysis by Moody et al the total complication rate after CLND was 37.3% including 21.6% of infection and delayed wound healing, 18% of lymphedema, 17.9% of seroma and 1.5% of hematoma. In terms of overall survival, White et al reported for CLND a rates of 43%, 35%, 28% and 23% at 5, 10, 15 and 20 years respectively. Concerning the SLNB, the complication rate has been reported at 10% in the MSLT-1 study consisting mostly of seroma/hematoma and infections. Lymph edema has been reported to occur in 1.5-1.7% of SNB cases [50-52]. In terms of overall survival, the technique was shown in patients with positive node a rates of 53% and 58.7% at 5 and 10 years respectively [53,54]. In our series, the complication rate was 28%, consisting mainly of lymphedema, Seroma, hematoma and skin necrosis. In order to reduce the incidence of these compliactions, actually we preserve during the procedure the saphenous vein which seems to improve the lymphatic drainage and realize muscle flaps with a view to to ensure local blood supply. In addition we will adopt prophylactic incisional negative pressure wound therapy which has proven itself in terms of efficiency and safety in terms of prevention of the major short and long-term postoperative complications following CLND procedure [55]. In our series, the survival rate was 45% and 17% at 3 and 5 years respectively (Table 1), which is much lower than those reported in the different series in the literature. We explain our low rate by our small number of patients and the frequent association in our context of inguinal and iliac metastases which considerably reduces survival, estimated at best at 20% at 5 years [56].
| Number of patient (n=18) | Age | Sex | Date of surgery | Last informations | Number of years lived since surgery | ||
|---|---|---|---|---|---|---|---|
| Date of the last consultation M/Y | State | Cause of death | |||||
| 1 | 39 | F | 01/2011 | 10/2017 | Alive | 6 | |
| 2 | 68 | H | 01/2011 | 02/2012 | Dead | Melanoma | 1 |
| 3 | 48 | H | 04/2011 | 06/2014 | Alive | 3 | |
| 4 | 50 | F | 06/2011 | 05/2013 | Alive | 1 | |
| 5 | 53 | F | 09/2011 | 01/2017 | Dead | Melanoma | 5 |
| 6 | 62 | F | 09/2011 | 03/2016 | Alive | 4 | |
| 7 | 44 | H | 10/2011 | 08/2017 | Alive | 5 | |
| 8 | 54 | F | 11/2011 | 02/2013 | Alive | 1 | |
| 9 | 66 | F | 01/2012 | 05/2015 | Alive | 3 | |
| 10 | 62 | H | 03/2012 | 05/2014 | Dead | Other | 2 |
| 11 | 50 | H | 03/2012 | 08/2017 | Dead | Other | 4 |
| 12 | 48 | H | 05/2012 | 09/2017 | Alive | 5 | |
| 13 | 46 | F | 06/2012 | 07/2016 | Alive | 4 | |
| 14 | 63 | F | 09/2012 | 11/2013 | Alive | 1 | |
| 15 | 68 | H | 11/2012 | 09/2015 | Dead | Melanoma | 2 |
| 16 | 54 | F | 11/2012 | 07/2014 | Dead | Other | 1 |
| 17 | 56 | F | 12/2012 | 09/2015 | Dead | Melanoma | 2 |
| 18 | 44 | H | 12/2012 | 10/2017 | Alive | 4 | |
The review of the literature allows us to retain that there is no significant differences in either melanoma-specific survival (MSS) and distant metastasis-free survival (DMFS) between patients who underwent CLND or positive SLNB [57]. This consolidates us in our therapeutic approach and encourages us in order to reduce the occurrence of postoperative adverse events to develop the endoscopic CLND.
The lymphatic spread of cutaneous melanoma worsens both disease-free and melanoma-specific survival. In this context the complete inguinal lymph node dissection improves local and regional disease and prevents the morbidity of pelvic dissemination, which is associated with poor quality of life. In order to reduce the incidence of its compliactions, this procedure must be practiced minutely by adopting the technical modifications which are described to it and by developing its endoscopic approach. In our daily practice, we adopt with great conviction the CLND for stage III cutaneous melanoma with a view to a prospective study with longer following up and a greater number of patients.
