Author(s): Yohanna Sorontou*and Agussalim*
Background: Falciparum malaria is a disease caused by Plasmodium falciparum which attacks humans throughout the world, especially in malaria endemic areas. In order to eradication the disease and avoid complications that may arise from severe infection. There is need to improve in management which includes evaluation of the current diagnostic methods. Diagnosis of malaria in resources limited and developing countries are commonly done by the detection of blood stages of the Plasmodium in Giemsa-stained blood smears by light microscopy. Blood smears are commonly prepared still until now using peripheral or venous blood.
Objective: To comparing the sensitivity of peripheral or venous blood for the detection density of malaria parasites based on ages group and gender.
Methods: Two blood smears were prepared from the peripheral and venous blood with EDTA from one patient, air dried, stained and examined following standard protocol by expert microscopic.
Results: Total samples of 40 patients, including. Parasite density of P. falciparum more than 10.000 per microliter in peripheral blood was found positive as much (25/40) slightly more rather than venous blood (21/40). Based on gender, peripheral blood of male as much (17/40) slightly more rather than in venous blood (14/40). The age group more than 20 years old, in peripheral was found positive as much (21/40) slightly more than in venous blood (16/40). Conclusions: Microscopic examination of malaria parasites will be more accurate using peripheral blood smears rather than venous blood with and without EDTA.
Falciparum Malaria is a disease that can cause death throughout the world, especially in development countries. According, estimates that there were 241 million malaria cases, including 627.000 deaths [1]. Worldwide in 2020 which represent around 14 million more cases and 68.000 more death than 2019 [1]. Children under 5 years of age are the group most likely to be affected by malaria in 2019 with a death rate of 67% as much 274.000 of all malaria deaths worldwide [2].
In 2010 positive cases of malaria in Indonesia reached 465.700,then in 2020 positive cases decreased to 235.700. Based on Annual parasite incidence in 2010 it reached 1.96 and in 2020 it reached1.87. Malaria cases in Indonesia increased to 304.601. The highest species of Plasmodium malarial is P. falciparum as much as 86.4% than to P. vivax 13.6% [3].
The current malaria morbidity rate and endemicity rate in Papua is very high and varies based on Annual Parasite Incidence 49/1000 population at the district/ city level in Papua. The highest malaria cases reaching 86.022 are still found in Papua and currently reach 90.9% [3].
The symptomatic of malaria is re-include cycles of chills, fever, sweats, muscle aches and headache that recur every few days with other symptoms such as vomiting, diarrhea, coughing and jaundice of the skin and eyes. Persons with severe falciparum malaria can develop bleeding problem, shock, kidney and liver failure, central of system nervous, coma and die P. falciparum main caused of human malaria infection. Falciparum malaria poses can be caused of several complicated and majority patients’ deaths [4,5].Venous blood is blood that is in the veins, which carries blood that lacks oxygen to the heart. Venous blood is drawn for routine blood testd or hematology.
EDTA is the typical anticoagulant used in blood collection tubes. It can be in a dry format or as a solution. The amount and concentration of EDTA require that blood should be collected up to a specific mark on the tube. If too little blood is collected, dilution of the sample can become an issue with alteration of parameters. Relative excess EDTA in such cases also affects the morphology of blood cell [6].
Purpose of this study was to determination of comparing the sensitivity of peripheral and venous blood using EDTA prepared smears for detection parasite density of falciparum malaria from speciment colection form randomly selected suspented of malaria in Jayapura general Hospital.
A total of 40 patients from Jayapura general Hospital was infected through Plasmodium falciparum. After we were doing an informed consent. Malaria screening was used microscopy. The patients presented at the clinical laboratory with fever in Jayapura General hospital.The diagnosis of malaria was the examination of thick and thin blood smears for malaria parasite by with Giemsa staining and finding parasite of Plasmodium falciparum with electric microscopy in clinical laboratory of Jayapura general hospital,we used the standard procedure. After the detection of malarial parasites thick blood smears were used to identify parasite density of Plasmodium falciparum and thin blood smears were used to identify the parasites species of Plasmodium [7,8].
For malaria parasite density. The number of asexual parasites per µl of thick and thin blood smears, will be calculated by dividing the number of parasites by the number of white blood cells counted and then multiplying by an assumed white blood cells density (8000 per µl) [7-9].
This study was doing at Jayapura general Hospital in Jayapura. Patients who seek treatment at Jayapura general hospital come from Jayapura district and City in papua Province, because this hospital is a referral hospital. Jayapura has aseason from January to April then rainfall decreases then rainfall then rainfall will increase again in Sepetember to December. Temperatur rata-rata 32 oC setiap tahun.Patients who were on antimalarial treatment within a month prior to the study were not eligible. Patients were required to give a written informed consent to the study which was duly explained to them in English and Indonesia. A was questionaire was administered to consented patients in order to obtain information on the demographic distribution of patients.
Two thick and thin blood smears were prepared from each patients using both perpheral and venous blood. perpheral blood was obstained using finger prick and venous blood was obstained from blood drawn into EDTA tubes. The blood smears were air,-dried and stained with 3% Giemsa during 45-60 minutes [9-11].
To detetion density of malaria parasite using a light electric microscopic,. The result examination was obstained from a third blood smears was invited to confirm the result and the results obstained by third microscopists were presumed [7]. The thick were screened for 200 fields using the 100 x ((with oil immersion) obyective. If density of malaria parasite were seen, the thin smear was then used to quantify parasitemia as well as were reached and the number determine the species of Plasmodium. The asexual stages of the Plasmodium were counted until 200 WBC were reached and the number obstained was divided by 200 and then multiplied by 8000 to give numbers in parasite per mm3 [7-9].
In thick blood preparations, the amount of blood is greater and lysis occurs during the process of making malaria preparations. Thick blood preparations consist of red blood cells with a larger amount of blood but the field of view is narrower, so the number of parasites is denser and easier to find. Erythrocytes are not visible due to lysis in the process of making malaria preparations, so malaria parasites will be concentrated in a limited area and will be found more quickly [7,9-11].
On a thin blood smear, the erythrocytes are still intact. You will see erythrocytes infected by malaria parasites. The shape of the erythrocytes is enlarged ot not, changed or not, there are Maurer spots or not, the spots are rough or smooth. Thin blood smears are used for mophological identification of plasmodium species because the erythrocytes are still intact and have one layer that is speread out to help indentify the morphology of the parasite, which is more clearly visible and can be caunted in number [8-11].
Examination of Giemsa stained blood smear using ligh microscopic is concidered the gold standard of diagnosis [12-14] Blood smears can be preapared using peripheral and venous blood with EDTA. The smears were also examined for staining characteristic of the smear as a whole and of malarial parasites of different stage species and the turnaround time and we were reporting in the result. In screening for density of malaria parasite, we used microscopic for evaluate each blood smears for its dilution staining patternm speed and case of reading the blood smears. The parasite density in the blood smears were caculate through counting the number of parasites per 200 white blood cell [15,16].
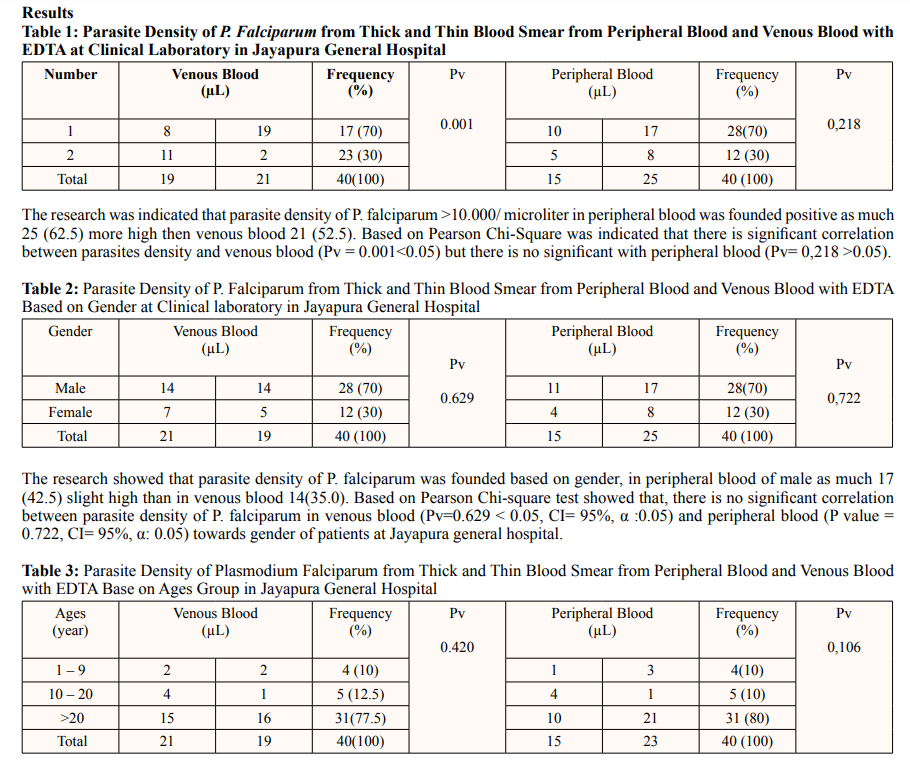
The research of this study was indicated that parasite density of P. falciparum in perpheral blood was found to be more than 10.000 parasites in aged more than 20 years as much as 21(10%) patients than venous blood.of 16(7.5%). Based on pearson chi-square test showed that, there is no significant correlation between the ages of the patient and parasite density of P. falciparum was founded in periphral blood (0.106 > 0.05) and venous blood (0.420 >0.05) towards ages of patients at Jayapura general hospital.
To determine the apropriate comparison of methods for examining malaria parasite density, we used peripheral blood samples taken from the fingerprick and venous blood with EDTA. There arises the need to optimize for diagnosis by the detection of parasite of P.falciparum in Giemsa stained blood smears by light electrical binocular microscopy. Blood smear for detection of P. falciparum parasites are commonly prepared fron peripheral and venous blood.
The result in this study showed that the density of P.falciparum parasites of more than 10.000 per mm3 detected through perpheral blood was found to be positive (25/40) slightly higher than positive venous blood (21/40). This is caused by parasites of P. falciparum has cytoadhere properties to taht it will stick to endothelium of perpheral or capillaries so that malaria parasites will be found more in the perpheral blood. The results of this study are the same as those found by observed, that red blood cells to cytoadhere to the endothelial cells lining blood vessels a feature associated with malaria pathology [17-20]. Whereas the taking venous blood for making thick and thin blood smears after administering the anticoagulant ADTA, the number of parasites are P.falciparum found will be fiwer in number or cannot be detected because the erythrocytes are lised and the blood becomes thin, the observed the same with Base on gender group, the paraste density of P.falciparum more than 10.000 per mm3 was found in men with positive peripheral smears (17/40) and venous blood (14/40) [14].
Whereas based on age group Base on ages group shown that density of P. falciparum > 10.000 has been found in men with positive perpheral as much as (17/40), (Pv =0.629) and venous blood (14/40), (Pv= 0.722). Whereas based on age group more then 20 years old, it was found positive in peripheral blood as much as (21/40), (Pv = 0.106) less high than venous blood (16/40), (Pv= 0.420). Base on Pearson chi-square test shown that, there is no significant correlation between peripheral and venous blood parasitemia. The result the same with [7,14] observed, no significant correlation was observed between capillary and venous blood parasitemia.
The result of this study indicate that the apropriate sampling method for examining falciparum malaria parasites in smaller quantities can be found in peripheral blood rather than at venous blood and it is not recommended to examine malaria parasites using venous blood with EDTA and especiaaly to assess the success of malaria treatment antimalarial efficacy test.
The author thanks to head of the clinical laboratry at Abepura general hospital and students of medical tecnology of laboratory who helped in taking samples.
Authors Contributions: This research was conducted in the clinical laboratory of Jayapura general hospital in May 2023. Taking venous and peripheral bloods, making preparations, drying and staining with Giemsa and washing the preparations and dying them and examining them under a microscope using immersion oil, writing and publishing.
Funding: This research received not external founding Institutional Review Board Statement: Applicable Informed Consent Statement: Applicable
Availability of Data and Material: The qualitative data supporting this study are not submitted. Participants did not consent to have their interview transcripts made submitted available.
