Author(s): A Marey* and Doaa F Ahmed
Innovative methods for handling industrial wastewater including heavy metals predominating covering technologies which has been a significant process as a new trend, consequently researchers owing to urge to promote an active method to eject toxic heavy metal ions from water bodies. Various materials act as very effective adsorbents such as our hydrogel that we synthesis in this study: Orange peel /Acrylamide composite hydrogel for removal of Co (II) from wastewater: surely derived adsorbent materials possess enormous concern. that research furthermore epitomizes several procedures parameters substantial to optimize adsorption ability followed by future research orientations.
As a material, hydrogel is extremely adaptable and may be used to a wide range of uses. Toxic or radioactive elements may be removed from a wide range of effluents using this material as an adsorbent, and metal pre-concentration can be used to examine environmental samples made from aqueous solutions. As a type of polymeric materials, the hydrogels can store enormous quantities of water in three-dimensional networks due to their hydrophilic structure [1]. It is critical that these products are widely used in a variety of industrial and environmental contexts. It is anticipated that synthetic hydrogels will be phased out over time because to their lower water absorption capacity, longer service life, and wider variety of basic chemical resources. Scientific fields, in particular, are doing an increasing amount of research on this subject. Since heavy metals tend to accumulate in living organisms and many are harmful or carcinogenic, the EPA lists them as priority pollutants [2]. The lungs, kidneys, central nervous system, nose, skin, and gastrointestinal tract are all affected by heavy metal ions pollution [3-5]. However, heavy metals continue to have an impact on the environment today. The situation has worsened in certain parts of the globe. Contamination of water resources due to the dumping of heavy metals has become a significant global problem in recent decades. Toxic or hazardous metals like chromium and antimony include copper, lead and mercury as well as cadmium and manganese [6].
In the winter, orange peel was collected from the surrounding area. Acryl amide (AAM) was purchased from win lab of purity (99%) and M.wt 71.08 a.m.u (UK). Hydrochloric acid and/or sodium hydroxide solutions were used to modify the pH as needed throughout the experiment. CoCl2.6H2O is a product of Sigma Aldrich’s. Almost all of the materials, including chemicals and reagents, have been used without being cleaned in any way. (OP) cleaned and rinsed with distilled water to eliminate any remaining material. When dry, the newly cleaned (OP) was crushed and sieved until the required particle sizes were achieved. Ismailia Canal provided the wastewater as effluent used in this investigation.: The turbidity (TSS) is (100.7) NTU, the pH is (8.32), and the temperature is (24.04oC) [7-9].
To make a homogeneous mixture with a 10% concentration, 100 ml distilled water was mixed with 10 g of (OP), swirled, and heated at 80°C for 120 minutes. (OP: AAM) [(1:1) (1:2), and (2:1)] monomers were continuously agitated at room temperature for 5 minutes as indicated in Figure1. The solution was then cooled to room temperature and various quantities of AAM were added. After being placed in a glass tube, the solution was exposed to the Co60 gamma radiation source at a range of dosage rates (10- 50 kGy) as well as a constant dose rate of 1.46 kGy/h. In order to eliminate any remaining unreacted chemicals, the irradiated crosslinked (AAM/OP) hydrogel was washed in excess water before being air dried at room temperature.
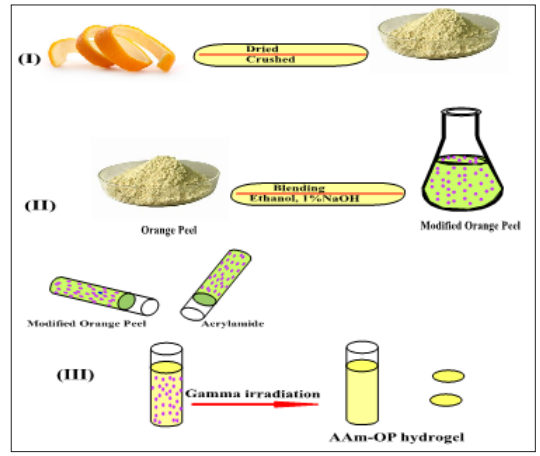
Figure 1: Formation OF AAm-OP Hydrogel
Radiation Preparation of (AAM/OP) Hydrogel Composite (AAM/OP) composite hydrogel was made by combining 0.1 g of orange peel with an equivalent amount of AAM monomer solution and stirring for 5 minutes. At room temperature, the viscous solution was placed into a glass tube and irradiated with Co60 gamma source, the vials were cracked and cut into discs with a thickness of 2 mm and a diameter of 5 mm, after the polymerization was completed [10]. The composite gel was rinsed with distilled water to remove any unreacted components before room temperature air-drying.
Figure 2 shows the initial Co (II) concentration on the hydrogel’s adsorption surface (AAM/OP). (AAM/OP) adsorbents removed a considerable percentage of cobalt ions at lower concentrations, with cobalt ion absorption by unit mass of the adsorbent being almost same in both cases. As the concentration of metal ions in the solution rises, so does the initial concentration of metal ions in the aqueous solution [11]. The percentage of Cobalt (II) absorption decreases fast as the initial concentration increases
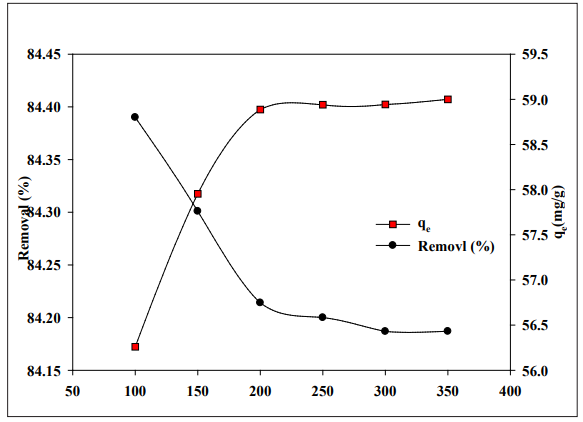
Figure 2: Influence of premier feed concentration of Cobalt ions on the adsorption of hydrogel at pH 6, contact time 48 h and at ambient temperature.
Figure 3 show linear isotherm plots of the Langmuir and the Freundlich model for adsorption of Co (II) onto the (AAM/OP) hydrogel, respectively, and the obtained data are summarized in Table 1. According to the correction coefficients of both isother.
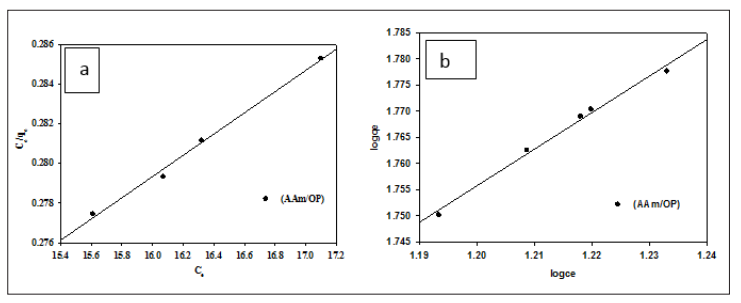
Figure 3: Adsorption of Cobalt ions onto A Produced Hydrogel using the (a) Langmuir Adsorption Isotherm (b) The Freundlich Adsorption Isotherm models, Co (II) adsorption fit The Freundlich isotherm model is superior to the Langmuir isotherm model in terms of accuracy.
|
Langmuir model |
Freundlich model |
||
|
Parameters |
(AAM/OP) |
Parameters |
(AAM/OP) |
|
qm (mg/g) |
186 |
KF [mg/g(mg/L) (-1/n)] |
8.2 |
|
ka (L/mg) ×10-3 |
3.3 |
1/n |
0.700 |
|
RL |
0.074 |
n |
1.42 |
|
R2 |
0.992 |
R2 |
0.998 |
The significance of contact duration between (AAM/OP) hydrogel and Cobalt (II) aqueous solution at pH = 6. Figure 4 shows the results of a study with an initial feed concentration of 100 mg/L. The elimination percent of Co (II) ions increases fast with contact time up to 20 minutes, when equilibrium is reached for the examined hydrogel. The concept that the Co (II) ions adsorption on the hydrogel is mostly due to electrostatic interactions is supported by the low contact time required to attain equilibrium [12-14].
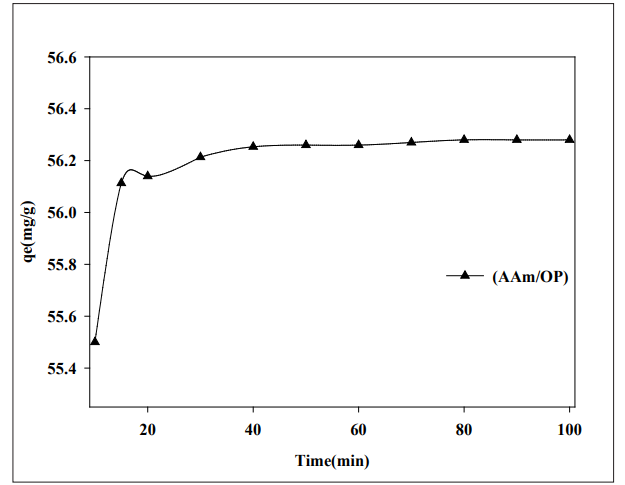
Figure 4: Impact of Contact Time on the Adsorption Capacity of Hydrogel for Cobalt Ions at pH 6, Initial Concentration 10 mg/l and at Ambient Temperature.
This research made use of pseudo-first and pseudo-second order kinetic models. coating the hydrogel with cobalt ions, as seen in Figure 5: chemical pathways (a, b). Adsorption of Co (II) onto an AAM/OP hydrogel may be described by a pseudo-second-order mechanism, as shown in Table 2, suggesting that the adsorption rate is controlled chemically. This is consistent with previous findings
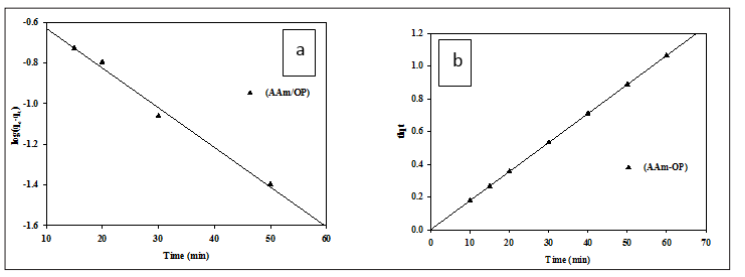
Figure 5: The kinetic plots of Cobalt Ions Adsorption onto Hydrogel via (a) Pseudo-First-Order Model, (b) Pseudo-Second- Order Model.
|
Pseudo-First Order |
Pseudo-Second Order |
||
|
Parameters |
(AAM/OP) |
Parameters |
(AAM/OP) |
|
q(ex) |
56.30 |
k2 (g/ mg min) |
0.183 |
|
k1 (min−1) |
0.0499 |
qe, cal (m g/ g) |
56.4 |
|
qe, cal (mg/ g) |
0.37 |
R2 |
0.999 |
|
R2 |
0.9845 |
|
|
Under various experimental circumstances, the elimination of Co (II) ions from wastewater using the created hydrogel was investigated utilizing Langmuir and Freundlich models for adsorption of Cobalt ions onto the researched hydrogel. The Freundlich isotherm model match the experimental data accurate. The rate of adsorption reduced as contact duration increased and a saturation stage was reached due to ions accumulation at the adsorption sites. This drop is owing to a reduction in the overall surface area of the adsorbent and an elevation in the diffusion. The adsorption of Co was discovered to be appropriate well with the pseudo-second-order model (II).
