Author(s): Caroline Delboni Nascimento, Ingrid Ardisson Coledete, Fernanda Nicoli Souza, Luiza Moraes Miossi, Luiza Rodrigues Moreira Julia Almenara Ribeiro Vieira, Caroline Tessinari Pupim and Renato Lirio Morelato*
ABSTRACT
Objectives: To analyze the associated cognitive frailty with falls and clinical admissions in older patients.
Methods: Case-control, analytical, individual-based, observational study based on longitudinal retrospective design. The sample comprised patients older than 65 years old, treated at the outpatient clinic of a geriatric service, who were followed up in the previous period of six months. Cognitive frailty (CF) was used as a dependent variable in the present study. Cognitive impairment was diagnosed based on Clinical Dementia Rating. Frailty was categorized based on the clinical frailty scale. The Chi-square test and Poisson regression model were used to evaluate the association between CF and outcomes.
Results: One hundred and forty-six patients, at a mean age of 81±6 years, were assessed; 69.2% of them were women. Patients with CF presented a higher risk of falls than the control population (p=0.004). However, after the adjusted analysis application, this condition was no longer associated with falls (p=0.32) and hospitalizations (p=0.59).
Conclusion: Patients with CF presented a higher risk of falls than the control population; however, this condition was not associated with hospitalization. These findings confirm the importance of strategies focused on preventing accidents due to falls, which could increase patients’ functional dependence on others.
Population aging is a reality worldwide. According to estimates, 25.5% of the Brazilian population will be older than 65 years by 2060 [1]. This process is associated with the emergence of age-related diseases and disabilities, with personal, social, and economic implications.
Dementia and frailty are often observed in individuals older than 75 years; these two conditions are closely related to each other [2]. Recent studies have suggested an association between these conditions and common physiological mechanisms such as chronic inflammation, impaired hypothalamic-pituitary axis stress response, imbalanced energy metabolism, mitochondrial dysfunction, oxidative stress, and neuroendocrine dysfunction [3,4].
The coexistence of physical frailty and cognitive impairment in patients who do not have dementia syndromes was defined as cognitive frailty by the International Academy of Nutrition and Aging (I.A.N.A.) and by the International Association of Gerontology and Geriatrics (I.A.G.G.) [5].
Population-based studies have estimated that the global prevalence of this syndrome ranges from 1% to 4.4% however, according to clinical-based studies, this condition presents a higher prevalence, ranging from 10.7% to 22% [1]. Concomitance between cognitive impairment and frailty reached 10.9% and 13.2% in two different studies conducted in Brazil [6,7].
Cognitive frailty has been associated with increased adverse outcomes in comparison to frailty syndromes and cognitive impairment, in separate. Among other outcomes, patients present reduced quality of life and functionality, as well as an increased number of hospitalizations, institutionalizations, falls and all cause mortality rates [8-12].
Based on a cross-sectional study, patients in the cognitive frailty group recorded significantly higher (48%) incidence of falls than patients in the robust (21%), physical frailty (34.5%), and cognitive impairment (22.2%) groups [10]. Longitudinal study and review have indicated that hospitalization does not correlate to cognitive frailty, but to physical frailty [2,13]. However, another recent meta-analysis has shown that cognitive frailty is a risk factor for all-cause hospitalization events [12].
Despite the growing interest in investigating the association between cognition and frailty, it is necessary to conduct further studies on this topic in developing countries. Therefore, the aim of the current study was to associate cognitive frailty with falls and hospitalizations in older patients followed up at a university geriatric outpatient service.
An analytical, case-control, individual-based, observational study based on the longitudinal retrospective design was carried out with patients with cognitive frailty (case group) and with individuals without cognitive frailty (control group). Participants were paired by age group, sex, weight, and schooling. The sample comprised patients older than 65 years old, treated at the outpatient clinic of a geriatric service of Santa Casa de Misericordia de Vitória philanthropic university hospital, Vitória City - ES, from August 2019 to February 2020, who were followed up in the previous period of six months. Participants who read and signed the informed consent form were included in the study. Patients with dementia of any etiology, with Parkinson’s disease, or those who were taking benzodiazepines, antidepressants, and neuroleptics were excluded from the study.
The sample size was calculated by taking into consideration the approximate cognitive frailty rate of 22% recorded in a longitudinal study carried out in France with approximately 300 patients with the neurocognitive disorder who were treated per semester, based on a sample error of 80% (type II error) at 5% significance level (type I error) and 95% confidence level [14]. Calculation results have shown that the approximate sample size in the current study should comprise 141 individuals (case/control) - in total, 146 patients were herein assessed.
Cognitive frailty was used as the dependent variable. Diagnostic criteria for this condition comprised simultaneous incidence of physical frailty and cognitive impairment diagnosed based on a Clinical Dementia Rating (CDR) scale of 0.5, without a concurrent diagnosis of Alzheimer’s disease or of other dementia types [5,15].
Rockwood’s clinical frailty scale - which addresses several patient- related domains and classifies patients into very fit, fit, managing well, living with very mild frailty, living with mild frailty, living with moderate frailty, living with severe frailty, living with very severe frailty and terminally ill - was used to assess frailty in the investigated population [16]. It was considered frail for those living with very mild frailty, living with mild frailty, living with moderate frailty, living with severe frailty, living with very severe frailty, and terminally ill. The aforementioned scale was included in the comprehensive geriatric evaluation routine of the investigated service. Patients’ frailty status was rated by the assistant doctor.
Age, sex, body mass index, marital status (single/divorced, married and widowed), schooling (literate, or not), independence for activities of daily living (Katz scale), functionality for the instrumental activity of daily living (Lawton & Brody scale), diagnosed comorbidities under clinical follow-up (systemic arterial hypertension, diabetes mellitus, congestive heart failure, and osteoporosis), blood pressure measured during medical consultation and routine laboratory tests of the service (blood glucose, vitamin B12, vitamin D, total cholesterol, triglycerides, creatinine, and blood count) were the herein analyzed covariables [17,18].
Mini-Mental State Examination (MMSE) was used for cognitive tracking purposes. Its total score reaches 30 points, and it is stratified by schooling, as follows: illiterate individuals (≥ 20 points); individuals with 1 to 4 years of schooling (≥ 25 points); 5 to 8 years of schooling (≥ 26 points); 9 to 11 years of schooling (≥ 28 points); and more than 11 years of schooling (≥ 29 points) [19].
Lawton & Brody’s scale was used to evaluate instrumental activities of daily living; its maximum score reaches 27 points, which corresponds to the highest independence level, whereas the minimum score is 9 points, and it corresponds to the highest dependence level [18]. Lawton and Brody’s scale are a reliable instrument used to assess individuals’ functional ability to perform instrumental activities of daily living in Brazil [20].
Katz scale comprises six items used to measure individuals’ performance in self-care activities [17]. The Portuguese version of the Katz scale of independence in activities of daily living was thoroughly developed and tested [21]. It was considered equivalent to the original version in English. Patients were herein classified into three categories, namely: 0 to 2 compromised domains, 3 and 4 compromised domains, and 5 and 6 compromised domains.
Outcomes corresponded to medical records of at least one fall event in the previous six months and hospitalization in the same period.
Continuous variables were expressed as the mean, standard deviation of the mean, and variability; whereas categorical variables were expressed in percentage (absent or present). A parametric or nonparametric test was used to compare means or medians after the evaluation of continuous sample distribution based on the Kolmogorov-Smirnov test - values p ≤ 0.05 rejected the null hypothesis of normality. Chi-square and continuous Student t or Mann-Whitney U tests - for parametric and nonparametric data, respectively - were used to compare categorical variables. Independent variables presenting a significance level of p ≤ 0.20 in the bivariate statistical analysis were included in the Poisson Regression model with robust variance in order to assess the association between the dependent variable and the outcomes, at 95% confidence interval.
P ≤ 0.05 was considered significant. The Statistical Package for the Social Sciences (SPSS) 27 software, licensed to the EMESCAM (Series: 10101141221) (Escola Superior de Ciencias da Santa Casa de Misericordia, Vitória, Brazil), was used to analyze the collected data. The research project was approved by the Research Ethics Committee of the School of Sciences of Santa Casa de Misericordia de Vitória, under number 3.225.605.
One hundred and forty-six patients, at mean age 81±6 (66-98) years, were included in the study; 30.1% (n=44) of them were men and 69.2% (n=102) were women, 57% (n=73) were illiterate and 91.78% (n=134) presented functional independence to perform basic activities of daily living - MMSE reached 21±4. Sample comprised 83 individuals (56.8%) in the control group and 63 individuals (43.2%) with cognitive frailty. Demographic features, blood pressure, comorbidities and laboratory tests of each group are shown in Table 1.
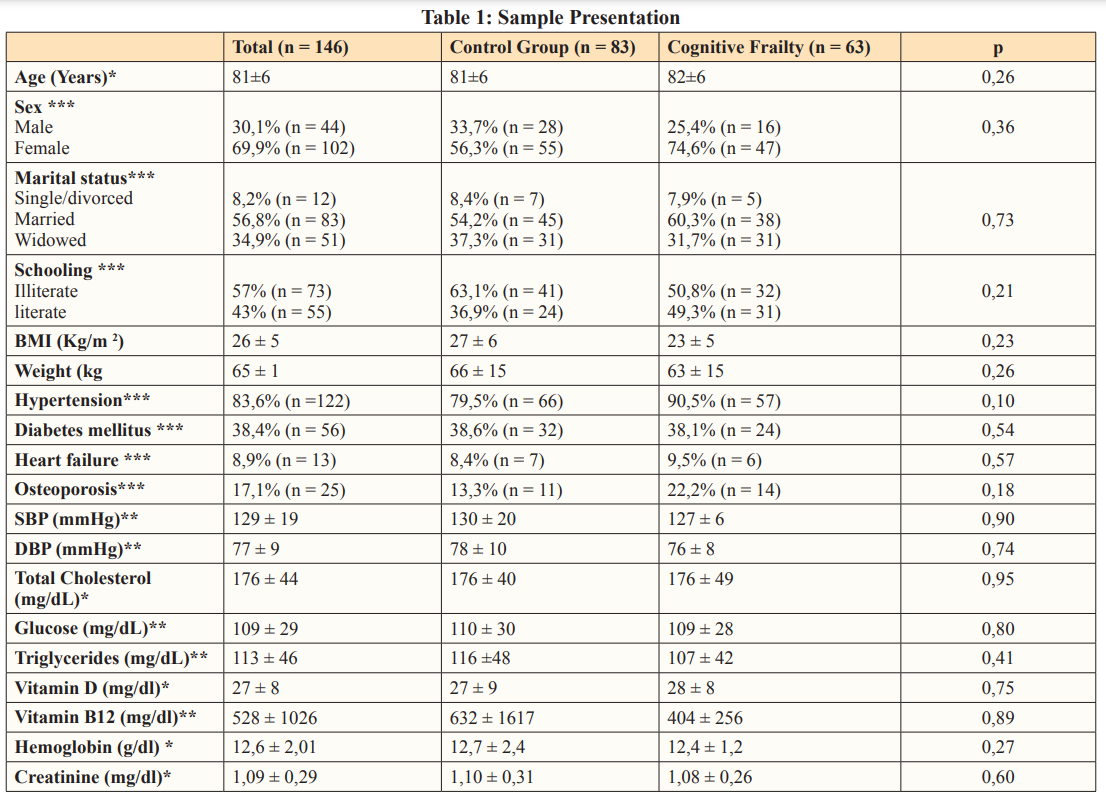
*Student’s t test **Mann-Whitney U test (non-parametric by the KS test) ***Chi-square test. BMI, body mass index; MMSE, mini mental state exam; SBP, systolic blood pressure; DBP, diastolic blood pressure; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; p, test significance.
The group with cognitive frailty was more dependent on others to perform instrumental activities of daily living than the control group (p < 0.001), as shown in Table 2. There was not significant difference in individuals’ dependence to perform basic activities of daily living between groups (p=0.06).
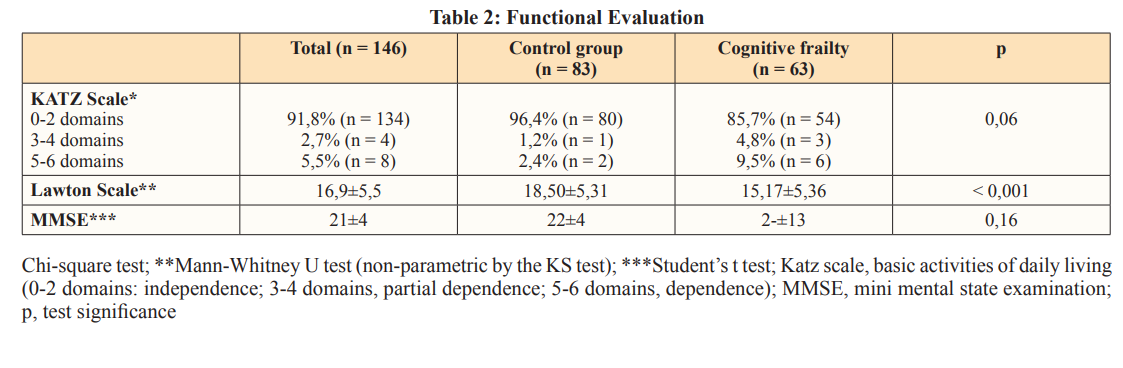
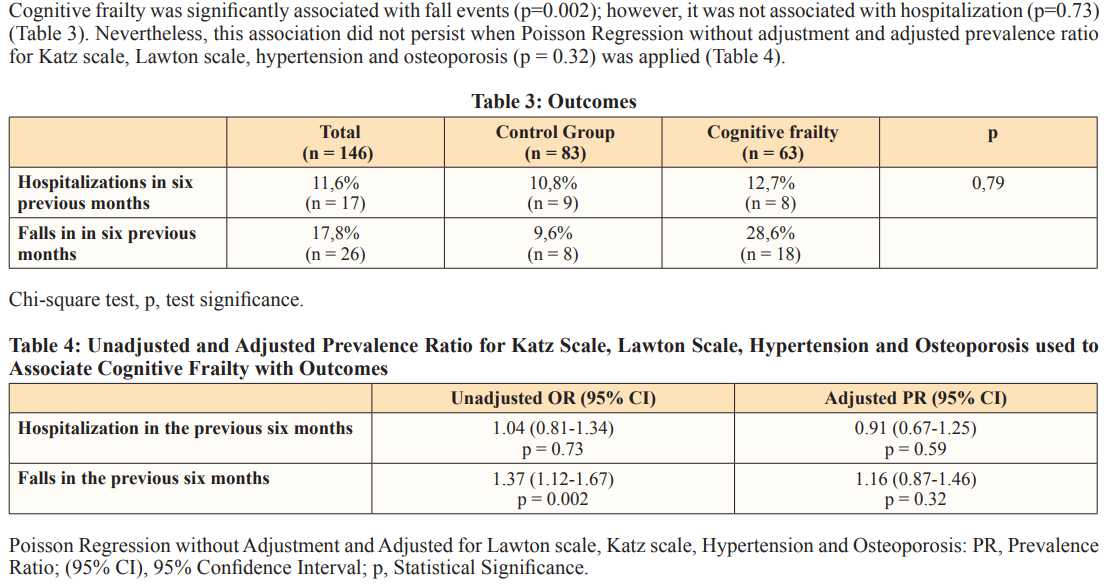
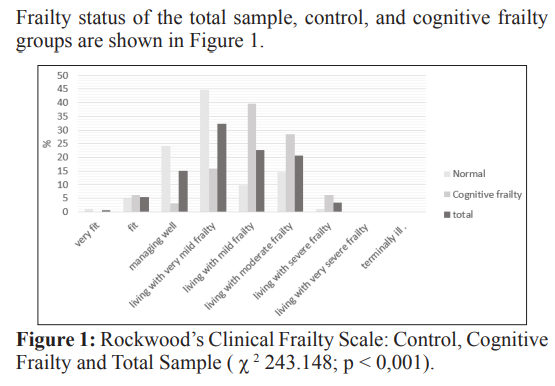
Patients with cognitive frailty presented a higher risk of falling than the control group; however, this association was not reported after the analysis was adjusted for Katz score, Lawton score, osteoporosis, and hypertension. Study conducted with the population living in the metropolitan region of Tokyo (Japan) has shown that history of fall events was significantly associated with cognitive frailty; moreover, the incidence of falls in this group was even higher than disability to perform instrumental activities of daily living [10]. This outcome strongly suggests that fall events might be a more serious concern [10,22]. Another Japanese study has shown that cognitive frailty was associated with fall events, as well as with fall-related fractures [8].
Cognitive frailty was not associated with hospitalization. Nevertheless, some studies have shown that this condition is a risk factor for all-cause hospitalization, as well as increases accumulated hospitalization time [12]. A study conducted with an older Chinese population living in Chicago has shown that patients with cognitive frailty were not just likely to undergo more hospitalizations, but they were also more than twice as likely to present one, or more, visits to the emergency department than those without cognitive and physical frailty [23].
Lower ability to perform instrumental activities may have contributed to the larger number of fall events in the cognitive frailty group. This group presented lesser functional independence and lower cognitive function, so it was more susceptible to presenting a reduced ability to predict the risk of daily accidents.
Patients with cognitive frailty presented a lower ability to perform complex activities of daily living. This outcome suggested greater cognitive dependence, although without influence on basic activities, in comparison to the control group. Rockwood and Theou have shown that dementia level often corresponds to physical frailty level. Thus, impaired ability to perform instrumental activities was observed in individuals with cognitive frailty. However, this outcome was not observed for basic activities of daily living, which can be explained by the fact that most patients had mild-to-moderate physical frailty [16].
With respect to functionality, a Malaysian cross-sectional study conducted with 815 patients suggested that a one-point reduction in the Katz scale has increased the risk of cognitive frailty by 2% [9]. Another cross-sectional population-based study has shown that this group had a higher risk of presenting limitations to performing instrumental activities of daily living and dependence [11].
Laboratory test results (glucose, cobalamin, vitamin D, total cholesterol, triglycerides, creatinine, and hematology markers) did not show significant differences between groups. Nevertheless, neuroinflammatory, nutritional, endocrine, cardiovascular, and hematology markers may suggest changes to the cellular immune system and hypothalamic-pituitary-adrenal axis, as well as increased risk of cognitive impairment, frailty, and death [3,4]. Besides, cobalamin is associated with cognitive functions through homocysteine metabolism and methylation reactions; however, the effects of subclinical levels on individuals’ cognition remain uncertain [24].
The cognitive frailty group recorded higher comorbidity, hypertension, heart failure, and osteoporosis rates than the control group, although without a statistically significant difference. Clegg et al, have shown that increased frailty was associated with a faster decline rate in several systems, even with cognitive decline [25].
Interventions such as chronic disease control (i.e., dyslipidemia, diabetes, and hypertension), fall prevention, smoking cessation, as well as engaging in an active and socially integrated lifestyle, exercising, and a healthy diet can delay cognitive frailty progression to dementia and prevent adverse events, such as disability, hospitalization, and death [2]. Thus, it is worth adopting an exercising routine and getting nutritional support, since these two aspects may account for reducing the influence of the pathology-disease ratio [26,27]. Accordingly, physical frailty precedes cognitive deficit in some cognitive frailty presentations; therefore, interventions aimed at improving cognitive frailty can avoid the development of cognitive disorders in the long term [2,25].
A recent systematic review has shown that performing dual-task exercises (combining physical activities and cognitive training) has significantly improved individuals’ cognitive functions, memory, and functional status [28]. However, other studies have compared multicomponent and dual-task exercises and concluded that both exercise types were effective in improving individuals’ physical and gait performance, although without the superiority of the second modality [29].
The current study has some limitations. It was a retrospective study conducted in a single service; therefore, causality became compromised. In addition, data were collected from medical records, which are susceptible to memory bias. Thus, it is necessary to conduct better-designed studies aimed at finding a better definition of cognitive frailty, as well as the means to enable the early prevention of adverse outcomes.
Patients with cognitive frailty presented a higher risk of falling than the control group; however, this association was not observed after adjustments based on Katz score, Lawton score, osteoporosis, and hypertension. Cognitive frailty was not associated with hospitalization. The cognitive frailty group has shown a lower ability to perform complex activities of daily living, a fact that suggested stronger cognitive dependence. These findings have confirmed the importance of developing strategies to prevent accidents due to falls, which could worsen individuals’ functional dependence.
1.Instituto Brasileiro De Geografia E Estatística. Projeções da População do Brasil e Unidades da Federação por sexo e idade: 2010-2060. Rio de Janeiro: IBGE, 2018. Disponível em: https://www.ibge.gov.br/apps/populacao/projecao/.
2.Panza F, Lozupone M, Solfrizzi V, Sardone R, Dibello V, et al. (2018) Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J Alzheimers Dis 62: 993-1012.
3.Ma L, Chan P (2020) Understanding the Physiological Links Between Physical Frailty and Cognitive Decline. Aging Dis 11: 405-418.
4.Sargent L, Nalls M, Starkweather A, Hobgood S, Thompson H, et al. (2018) Shared biological pathways for frailty and cognitive impairment: A systematic review. Ageing Res Rev 47: 149-158.
5.Kelaiditi E, Cesari M, Canevelli M, Van Kan GA, Ousset PJ, et al. (2013) Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging 17: 726-734.
6.Brigola AG, Ottaviani AC, Alexandre TDS, Luchesi BM, Pavarini SCI (2020) Cumulative effects of cognitive impairment and frailty on functional decline, falls and hospitalization: A four-year follow-up study with older adults. Arch Gerontol Geriatr 87: 104005.
7.Brigola AG, Ottaviani AC, Carvalho DHT, Oliveira NA, Souza EN, et al. (2021) Association between cognitive impairment and criteria for frailty syndrome among older adults. Arquivos de Neuro-Psiquiatria 78: 2-8.
8.Tsutsumimoto K, Doi T, Makizako H, Hotta R, Nakakubo S, et al. (2018) Cognitive Frailty is Associated with Fall- Related Fracture among Older People. J Nutr Health Aging 22: 1216-1220.
9.Malek Rivan NF, Shahar S, Rajab NF, Singh DKA, Din NC, et al. (2019) Cognitive frailty among Malaysian older adults: baseline findings from the LRGS TUA cohort study. Clin Interv Aging 14: 1343-1352.
10.Kim H, Awata S, Watanabe Y, Kojima N, Osuka Y, et al. (2019) Cognitive frailty in community-dwelling older Japanese people: Prevalence and its association with falls. Geriatr Gerontol Int 19: 647-653.
11.Shimada H, Makizako H, Lee S, Doi T, Lee S, et al. (2016) Impact of Cognitive Frailty on Daily Activities in Older Persons. J Nutr Health Aging 20: 729-735.
12.Bu Z, Huang A, Xue M, Li Q, Bai Y, et al. (2021) Cognitive frailty as a predictor of adverse outcomes among older adults: A systematic review and meta-analysis. Brain Behav 11: e01926.
13.Chen C, Park J, Wu C, Xue Q, Agogo G, et al. (2020) Cognitive frailty in relation to adverse health outcomes independent of multimorbidity: results from the China health and retirement longitudinal study. Aging (Albany NY) 12: 23129-23145.
14.Delrieu J, Andrieu S, Pahor M, Cantet C, Cesari M, et al. (2016) Neuropsychological Profile of “Cognitive Frailty” Subjects in MAPT Study. J Prev Alzheimers Dis 3: 151-159.
15.Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43: 2412-2414.
16.Rockwood K, Theou O (2020) Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can Geriatr J 23: 210-215.
17.Katz S, Chinn AB, Cordrey LJ, Grotz RC, Newberry WB, et al. (1959) Multidisciplinary studies of illness in aged persons.
II. A new classification of functional status in activities of daily living. J Chronic Dis 9: 55-62.
18.Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9: 179-186.
19.Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH (2003) Sugestões para o uso do mini-exame do estado mental no Brasil [Suggestions for utilization of the mini- mental state examination in Brazil]. Arq Neuropsiquiatr 61: 777-781.
20.dos Santos RL, Júnior JSV (2008) Confiabilidade da versão brasileira da escala de atividades instrumentais da vida diária. Rev Bras Promoç Saúde 21: 290-296.
21.Lino VTS, Pereira SRM, Camacho LAB, Ribeiro Filho ST, Buksman S (2008) Adaptação transcultural da Escala de Independência em Atividades da Vida Diária (Escala de Katz). Cad Saúde Pública 24: 103-112.
22.Ma Y, Li X, Pan Y, Zhao R, Wang X, et al. (2021) Cognitive frailty and falls in Chinese elderly people: a population-based longitudinal study. Eur J Neurol 28: 381-388
23.Wang J, Kong D, Yu F, Conwell Y, Dong X (2021) Cognitive deficit, physical frailty, hospitalization and emergency department visits in later life. Aging Ment Health 25: 521-527.
24.Smith AD, Refsum H (2009) Vitamin B-12 and cognition in the elderly. Am J Clin Nutr 89: 707S-711S.
25.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381: 752-762.
26.Liu Z, Hsu FC, Trombetti A, King AC, Liu CK, et al. (2018) Effect of 24-month physical activity on cognitive frailty and the role of inflammation: the LIFE randomized clinical trial. BMC Med 16: 185.
27.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, et al. (2019) Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol 18: 177-184.
28.Law LL, Barnett F, Yau MK, Gray MA (2014) Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: a systematic review. Ageing Res Rev 15: 61-75.
29.Rezola-Pardo C, Arrieta H, Gil SM, Zarrazquin I, Yanguas JJ, et al. (2019) Comparison between multicomponent and simultaneous dual-task exercise interventions in long-term nursing home residents: the Ageing-Ondual-Task randomized controlled study. Age Ageing 48: 817-823.
View PDF