Author(s): Shailja Rangra, Prem Lata, Kiran Bala Sharma, Reena Kumari and Savitri*
The present study utilized computational-guided experiments to conduct screening of a novel discovered acidic-thermostable α-amylase (AMYT) derived from a metagenome of hot springs. This approach was employed as a cost-effective alternative to the conventional method of functional screening. Initially, a computational screening methodology was employed to identify primary candidate that possess superior properties. AMYT was subjected to cloning, expression, purification, and characterization, among the candidates that were chosen. The AMYT enzyme demonstrated optimal activity at pH of 5.5 and a temperature of 50 °C. The enzyme demonstrated high efficacy in the presence of diverse chemicals, demonstrating a remarkable capacity in the hydrolysis of a wide range of substrates. Furthermore, it was found to be independent of Ca2+ ions. The results of this study demonstrated the efficacy of computational methods in identifying previously unknown acidic thermostable α-amylases. The accuracy of the selection method suggests that the AMYT has the potential to be a viable option for industrial starch processing, as it has the ability to enhance the output of final products and decrease overall costs.
Amylases represent a diverse group of hydrolase enzymes that account for approximately 25% of the overall enzyme market sales. One of the essential constituents of this group is α-amylases (EC 3.2.1.1). These endo-acting enzymes hydrolyze the α-1,4-glycosidic linkages present in starch, resulting in the formation of glucose, maltose, maltotriose, and low molecular- weight dextrins. α-Amylases belong to the glycosyl hydrolase family 13 and have been studied across a range of organisms, including plants, mammals, and microorganisms [1]. The extensive utilization of amylolytic enzymes across various industries, such as pharmaceuticals, detergents, textiles, paper, alcohol production, brewing, and starch processing, has demonstrated their remarkable potential as additives and supplements [2].
Despite the various sources such as bacteria, fungi, and yeasts for the generation and characterization of novel α-amylases, advancements in practical techniques have emerged to better achieve this objective [3]. The conventional methods reliant on culture-based techniques account for less than 1 % of the overall microbial diversities. Through the implementation of a functional methodology, metagenomics enables the identification of microbial communities that are not amenable to cultivation using other approaches [4]. Furthermore, this particular approach has proven effective in the identification of antibiotics, enzymes, antimicrobial compounds, and various pathway genes. Novel amylases exhibiting distinctive characteristics were identified in various metagenomic libraries, including soil, rumen fluid, hot springs, acid mine drainage, and fecal microbial samples, with the aim of exploring their potential utility in diverse applications [5]. Nowadays, there is a significant emphasis on α-amylases that demonstrate remarkable thermal stability, due to their extensive utility in diverse industrial processes. These enzymes are mostly derived from thermophilic microorganisms. They offer significant advantages in terms of reducing cooling costs, enhancing substrate solubility, improving diffusion rates, reducing microbial impurities, and maintaining stability against denaturing agents [6].
The utilization of thermostable α-amylases in the processes of starch hydrolysis, liquefaction, and saccharification has exhibited substantial potential for application in the food industry and starch-based sectors. Other specific characteristics of α-amylase enzymes in relation to their utilization in starch-based applications include their resilience in the face of oxidative stress conditions and their ability to function independent of calcium ions. These characteristics facilitate the improved transformation of starch into various sugar syrups [7].
Metagenomics refers to the methodology employed for the analysis of an environmental sample in a culture-independent manner. This approach combines molecular biology and genetics to extract, identify, characterize, and make use of the vast majority of genetic information present within the sample [8]. Therefore, this study aimed to computationally analyze the metagenome data of hot springs in order to identify suitable candidate enzymes with acidic and thermostable α-amylase properties. Subsequently, the AMYT gene was cloned, expressed, purified, and subjected to characterization. The enzymatic properties of this particular enzyme are noteworthy due to its thermo-stability and acid- stability, which are considered crucial factors in the context of industrial starch processing.
The SignalP-6.0 webserver was used to determine the potential presence of a signal sequence in the AMYT protein [10]. The Clustal Omega tool was employed to perform a multiple sequence alignment using proteins that had been previously characterized [11]. The ExPASy protparam tool was utilized to evaluate the theoretical molecular mass and isoelectric point (pI) of the AMYT protein. The evolutionary relationship of AMYT protein was determined by constructing a phylogenetic tree using the
Molecular Evolutionary Genetics Analysis (MEGAX) software, which utilized protein sequences of previously identified amylases from various origins as input [12]. This study employed the Neighbor-Joining method, utilizing 1000 bootstrap replications with poison adjustments. The reference crystal structures of consensus sequences submitted in the PDB database were used for protein structure prediction. The SWISS-MODEL was used to conduct Ramachandran plot and homology modelling of AMYT protein [13, 14].
Cloning, Expression and Purification of α-amylase Metagenomic DNA from Tattapani thermal spring was taken as the template for the amplification of the putative α-amylase gene (amyT). The full coding sequence of amyTwas amplified by polymerase chain reaction using Q5 high fidelity DNA polymerase (NEB, MA, USA) and forward primer (5′-CGC GCG GCA GCC ATA TGA TGA GGC TTT TGC ATC TGG AGC-3′ with NdeI restriction site and reverse primer (5′-GGT GGT GGT GCT CGA GTC ATC TCA CCA GGG TCC AGA CC-3′) with XhoI restriction site. For expression of the recombinant protein, pET- 28a(+) plasmid containing the enzyme gene was transformed into BL-21 (DE3) competent E. coli cells, and correct insertion was confirmed by sequencing. Recombinant E. coliBL21- pET- 28a(+)-amylase was cultivated at LB medium supplemented with kanamycin (50 μg/ml) at 37 °C with shaking (180 rpm) until the absorbance at 600 nm reached approximately 0.6.Then, isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5 mM for 4 h at 37 °C, followed by mM NaH2PO4, 20 mM imidazole; pH 8) the column was washed multiple times. The desired recombinant protein (AMYT) was eluted with an increasing gradient of 50-350 mM imidazole in elution buffer (50 mM NaH2PO4, pH 8) and its concentration was measured by the Bradford method [15]. The protein samples were evaluated for purity and homogeneity by running 12 % of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and followed by Coomassie brilliant blue G-250 staining [16].
For determination of α-amylase activity, DNSA method was followed, taking glucose as the standard [17]. The standard enzyme assay was performed using reaction mixture, containing enzyme of required concentration and starch (1% w/v) in sodium acetate buffer (50 mM) of pH 5.5 as substrate. The reaction mixture was subjected to incubation at a temperature of 60 °C for 10 min. After incubation, reaction was stopped with the addition of equivalent amount of freshly prepared 3, 5-dinitrosalicylic acid (DNSA) reagent. After addition of DNSA, the mixture was again heated at 100 °C for 10 min. The mixture of distilled water (equivalent to the volume of reaction cocktail) and DNSA reagent was heated simultaneously and taken as the blank control. The heated components were allowed to cool at room temperature followed by absorption measurement at 540 nm wavelength. The amount of enzyme required to release the reducing sugars (equivalent to 1 μmol of glucose) per min under standard assay conditions was considered as one unit (IU) of enzyme activity.
Various reaction parameters that are crucial for enzyme activity were optimized for the purified enzyme and assays were performed in triplicates. The enzyme assay was conducted using the standard protocol outlined in the preceding section.
The effect of temperature on the activity of AMYT protein was examined by carrying out standard enzyme assays at varied temperature, ranging from 10-80 °C. Moreover, to examine the effect of different buffers and their respective pH on enzyme activity, the enzyme assays were performed with different buffers in their efficient working pH ranges, i.e. acidic range buffer, such as glycine HCl (pH 2.0-3.0) and sodium acetate buffer (pH 3.5-6.0), sodium phosphate buffer of neutral range (pH 6.0-8.0), basic range buffers, such as tris-HCl (pH 8.0-9.0), and glycine NaOH (pH 10.0).
In order to study the effect of various metal salts (CaCl2, FeSO4, MgCl2, MnSO4, CoCl2, CuSO4, CdCl2, ZnCl2, KCl and NaCl) in their ions form (Ca2+, Fe2+, Mg2+, Mn2+, Co2+, Cu2+, Cd2+, Zn2+, K+,and Na+) on the activity of AMY , the enzyme was pre-incubated with each of the selected salt-ions (1 mM) for 30 min at room temperature.
To examine the effect of detergents and inhibitors on the activity of AMYT was investigated. The detergents were tested by incubating enzyme with 0.5 % w/v each of Triton X-100, Tween 20 and SDS and inhibitors used are EDTA and PMSF each at a concentration of 1 mM. Each additive was preincubated with the enzyme for 30 min at room temperature. The amylase activity was assessed using standard conditions. The above-mentioned experiments involved the measurement of relative activities under enzyme assay conditions, with the activity in the absence of any additives being designated as the control (100 %).
The influence of different pH and temperatures on the stability of purified amylase was examined. For temperature stability, aliquots of purified proteins stored in the buffer were subjected to different temperatures from 30 °C to 80 °C for a varying intervals of time. Following this, the standard enzyme test was used to measure the residual activity of the enzyme fractions exposed to heat. For pH stability profiling, AMYT was pre-incubated in the buffer of pH 4.0-9.0 for 4 h and measured by the residual activity.
The relative activity of amylase was examined on various substrates including wheat starch, potato starch and corn starch, glycogen, and pullulan at 1 % w/v concentration. The enzymatic assay was performed for each substrate.
Gene Identification and Sequence Analysis of amyTGene The hot spring metagenomic data were examined to identify a new gene encoding an α-amylase. A metagenomic DNA fragment (amyT) with a length of 1962 bp was predicted to encode a protein with amylase activity on the basis of sequence similarity analysis. The anticipated α-amylase, AMYT, was shown to have primary sequence similarity to the protein of glycosyl hydrolase family in the CAZy database. BLASTn and BLASTx analysis of amyTsequence against NCBI nr database at default parameters, show result with putative α-amylase of Methanothrix harundinacea6Ac, still uncharacterized. This indicated the novelty in the genomic source of the α-amylase identified in the present study. The conserve domain analysis and motifs prediction using CDD depicted the presence of the conserved domain (amino
acid residues 133-550) for α-amylase’s catalytic activity. The residues, Asp348, Glu408, and Asp438, are vital for the catalytic utility of the protein [18, 19]. The previously characterized amino acid sequences of known α-amylases together with AMYT, were used for analyzing the sequence features and determining the phylogenetic tree. According to phylogenetic analysis, based on homologous protein sequences, amyTfound to be evolutionarily closer to AAF17100.1 alpha-amylase (Aspergillus nidulans) and AEH03024.1 alpha-amylase (Aureobasidium pullulans) as shown in Figure 1. The nucleotide sequence of amyTwas deposited in the GenBank database under accession number OR193746.
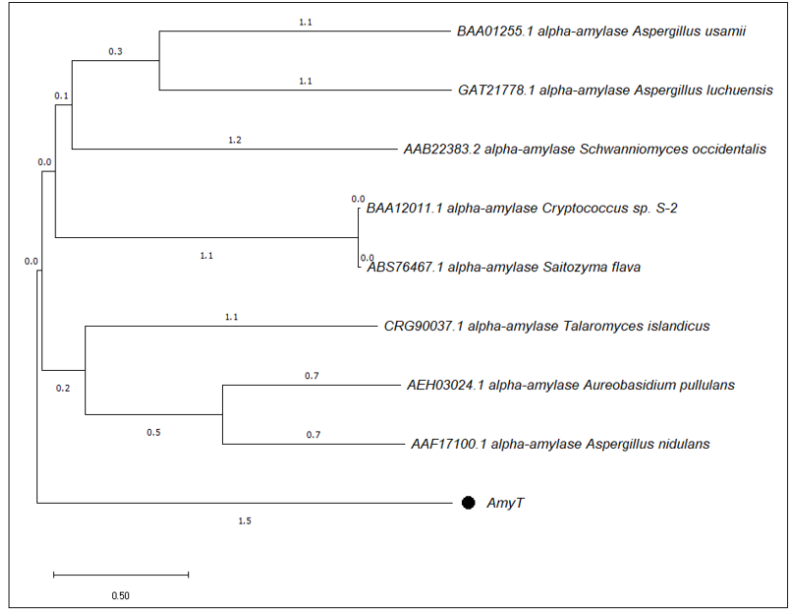
Figure 1: Cladogram Showing Phylogenetic Relatedness of AMYT with other Homologous Protein Sequences
The multiple-sequence alignment of AMYT with previously characterized α-amylases revealed the conserved residues (Figure 2), presumably essential for catalytic activities of the protein.
The arginine (Arg) residues contributes to stability in thermally adapted enzymes, since it is capable of forming more than one salt bridge and up to five hydrogen bonds [20]. The theoretical molecular mass of AMYT was estimated to be about 74 kDa, and isoelectric point (pI) of 5.32. There was no signal peptide identified in AMYT protein. According to earlier research, signal peptide is not required for proper folding of the α-amylase [21].
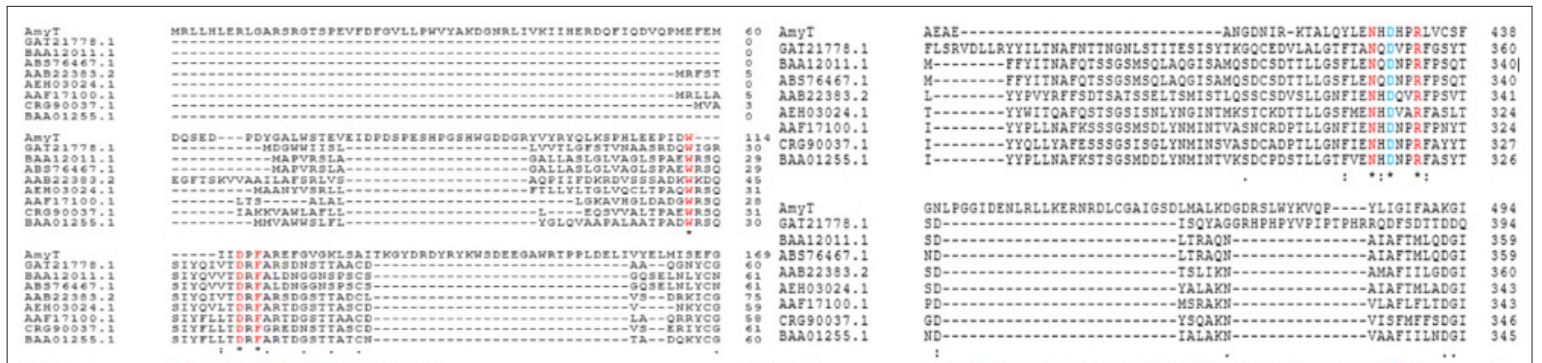
Figure 2: Multiple sequence alignment of AMYT with other characterized α-amylase (Aspergillus luchuensis, GAT21778.1; Cryptococcus sp. S-2, BAA12011.1; Saitozyma flava, ABS76467.1; Schwanniomyces occidentalis, AAB22383.2; Aureobasidium pullulans, AEH03024.1; A. nidulans AAF17100.1; Talaromyces islandicus, CRG90037.1; A. usamii, BAA01255.1). The highlighted sequences in blue and red color indicate conserved residues in Gh43 family.
Homology modeling of AMYT was done using SWISS-MODEL. The putative enzyme sequence was modelled using α-amylase of Methanotrichaceae archaeon (A0A7J4PR98.1.A), exhibiting a sequence identity of 76.37 %. Ramachandran plot analysis showed >95 % amino acid residues in favored region (Figure. 3a), and 0 % were predicted to be Ramachandran outliers. The putative model of α-amylase showed the presence of α/β-sheets in the modelled structure (Figure. 3b). The crystal structure of Methanotrichaceae archaeon (A0A7J4PR98.1.A) served as a basis for the homology model of AMYT (Figure. 3c).
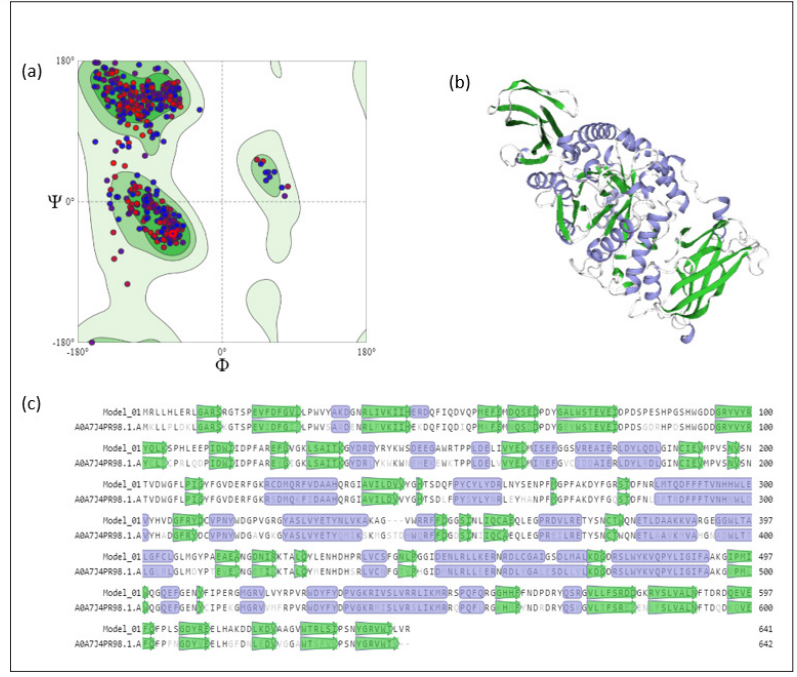
Figure 3: (a) Ramachandran Plot Depiction of AMYT Showing maximum Residues under the Allowed Region (b) Three-Dimensional Homology Model of AMYT (c) Secondary Structure Prediction in AMYT Revealing Residues Involved in Parallel β-sheets (arrows) and Turns (purple)
The amyT gene was cloned in pET-28a(+) vector followed by expression in E. coli BL21 (DE3) cells. The heterologous expression of amyT gene was investigated in E. coli BL21 (DE3) via IPTG induction. The concentration of 0.5 mM IPTG and 37 ºC temperature was noted to be relatively more favourable for gene expression. In addition, AMYT protein purified by Ni-NTA affinity chromatography was subjected to SDS-PAGE electrophoresis. The SDS-PAGE analysis indicated >90 % purity in the purified fraction of the recombinantly expressed AMYT protein. The protein concentration was estimated using Bradford procedure. SDS-PAGE determined the apparent molecular mass of protein to be approximately ~85 KDa (Figure. 4), which is in accordance with the theoretically predicted molecular mass of AMYT. This is in agreement with the molecular mass of the monomeric α-amylase reported from Anoxybacillus sp. Ah4 Bacillus licheniformis AT70 and Bacillus halodurans MS-2-5 [22-24].
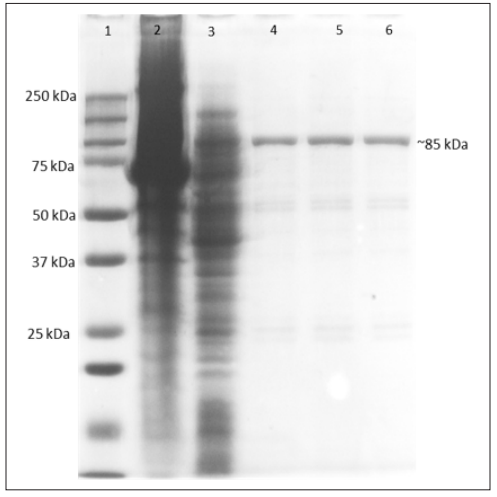
Figure 4: SDS-PAGE Analysis of AMYT Expressed in E. coli. Lane 1: SDS-Protein ladder; Lane 2: Cell Pellet; Lane 3: Crude enzyme; Lane 4, 5, 6: Purified protein.
The temperature optima for the AMYT protein was estimated by performing the enzyme assay in the temperature range of 10-80°C. AMYT showed maximum enzyme activity at 50 °C. However, more than 65 % relative activity was seen in a temperature range of 40-60 °C (Figure 5a). The α-amylase reported from Aspergillus oryzae IFO-30103 and Bacillus alcalophilus JN21 have similar temperature optima as that of AMYT [25, 26]. The high activity under high temperature makes AMYT a suitable candidate to use for starch hydrolysis into starch hydrolysates such as glucose and fructose [27]. In pH profiling, AMYT protein showed the maximum activity at pH 5.5 in 50 mM of sodium acetate buffer. The extreme pH conditions of 2.0 and 10.0 severely hampered the AMYT activity. The AMYT protein maintained more than 60 % activity at pH 5.0-7.0, more than 40 % was retained at pH 8.0-9.0, although the amylase activity under extreme pHs dropped rapidly (Figure 5b). The same result was observed for Bacillus velezensis KB 2216 (pH 5.5), Aspergillus oryzae IFO-30103 (pH 5.5), Bacillus YX-1 (pH 5.5), Penicillium olsonii (pH 5.5) [28-31].
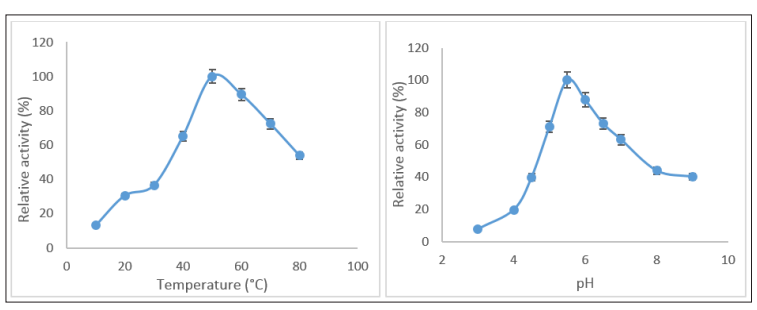
Figure 5: (a) Effect of pH on AMYT Activity (b) Effect of Temperature on AMYT Activity.
Effect of Metal ions, Inhibitors and Surfactants on AMYT The effect of different metal ions (Ca2+, Fe2+, Mg2+, Mn2+, Co2+, Cu2+, Cd2+, Zn2+, K+, and Na+) was determined on the activity of AMYT enzyme at 50 ºC. The AMYT activity demonstrated a slight increase when exposed to K+, Na+, Mn2+ and Ca2+, while it exhibited a moderate decrease in the presence of Mg2+ and Fe2+.
Generally, Mn2+ is included in the enhancement of α-amylase activity [32]. However, in the present study, Mn2+ and Ca2+ did not influence the amylase activity. Nevertheless, the enzyme activity significantly decreased in the presence of Cu2+ and Cd2+ (Figure 6a). Thermostable α-amylases from Geobacillus thermoleovorans and B. subtilis JS-2004 showed the same results towards tested metal ions [33, 34].
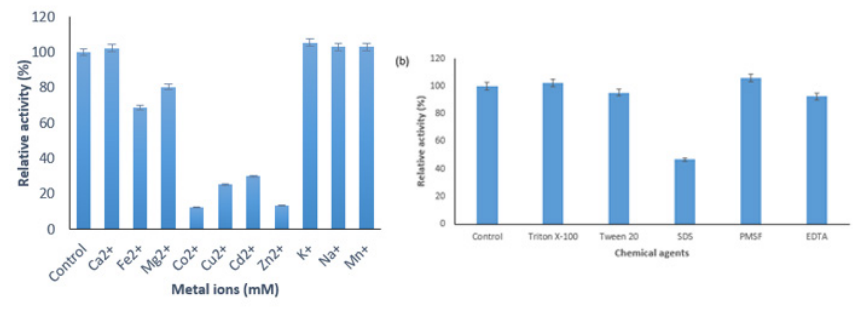
Figure 6: (a) Effect of Metal ions on the Activity of AMYT (b) Effects of chemical agents on AMYT activity The effect of various inhibitors and surfactants on the activity of AMYT protein was checked (Figure 6b). AMYT protein was affected in the presence detergents (Triton X-100, Tween 20, and SDS) and inhibitors (EDTA and PMSF). AMYT protein showed remarkable resistance towards different inhibitors including Triton 0, Tween 20 and PMSF. The observable activity of AMYT activity in the presence of EDTA indicated that this inhibitor might not be acting as a chelating agent for AMYT which is consistent with previous studies [35, 36].
Thermostability and pH stability profiling of AMYT Thermostability profiling of AMYT protein indicated remarkable storage stability at 30 °C and 40 °C, with >60 % residual activity after 4 h of incubation. Under the exposure of 50 °C and 60 °C, the enzyme retained 50 % residual activity after 4 h. In comparison to other tested temperatures, the protein was found to be least stable at 70 °C and 80 °C, with 50 % residual activity after 4 h (Figure 7a). Compared with some amylases, such as Bacillus subtilis N8 with 83 % activity after 60 min at 40 ºC, Pseudoalteromonas M175 <10 % activity after 60 min at 50 ºC, Bacillus methylotrophicus <60 % activity after 60 min at 50 ºC, Bacillus pseudofirmus <80 % activity after 20 min at 50 ºC, metagenome-derived <10 % activity after 60 min at 50 ºC, AMYT showed remarkable thermostability [37-41].
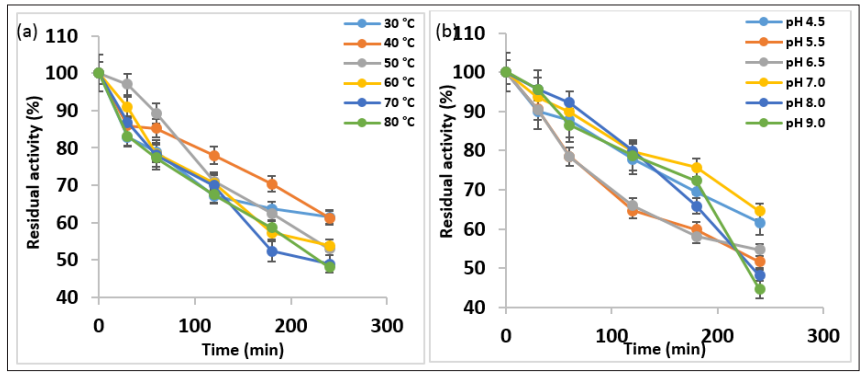
Figure 7: (a) Thermostability Profile of AMYT Protein; (b) pH Stability Assessment of AMYT
The pH stability profiling of AMYT protein emphasized it as a stable protein under a wide pH range of highly acidic to moderately alkaline range. The protein was found to be highly stable under pH 4.5 and 5.5, retaining more than 50 % residual activity after 4 h of incubation. However, the protein remained substantially active when incubated under pH 6.5 and 7.0 for 4 h. The protein was found to be least active after 4 h of incubation at pH 8.0 and 9.0 (Figure 7b). The use of acid stable amylase will omit expensive and time consuming step of adjusting pH to 4.5 during the saccharification process and the formation of by-products will be reduced [42]. Consequently, this enzyme possesses the favorable activity under acidic and high temperature conditions compared with the amylases reported in the literature, which will provide a better choice for enzymatic starch processing.
Enzyme catalyzed reactions are also affected by the type and concentration of substrate. The affinity of AMYT was evaluated towards different substrates, i.e. wheat, potato and corn starch, glycogen, and pullulan. The affinity of AMYT was found to be maximum towards potato starch (Figure 8). It also shown the significant activity on pullulan with α-1,6 linkages which signified the broad range substrate activity of AMYT. The ability of AMYT with thermal stability in acidic conditions to hydrolyze various starches was reported before, and it was introduced as a great candidate for utilization in starch industry [43].
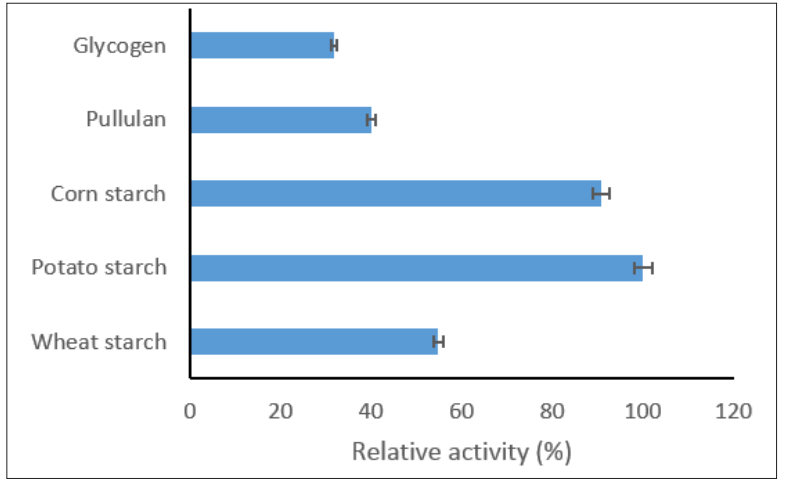
Figure 8: Relative Activity of AMYT in the Presence of Different Substrates
This study aimed to investigate the hot spring metagenome through computational screening in order to identify a novel acidic-thermostable α-amylase. The primary benefit to utilize computational approach procedure was to refine a vast amount of potential α-amylase and narrow down the list of candidates to a minimal number of enzymes with superior desired properties, in contrast of functional metagenomics screening methods. After identification, cloning, expression, purification, and characterization of AMYT was experimentally done. The AMYT was able to retain more than 70 % of its maximum activity after 60 min at 80 °C and was optimally active at pH 5.5 and temperature of 50 °C. The stability of the α-amylase enzyme was demonstrated by its appropriate activity in the presence of multiple additives and diverse substrates. The results showed the excellent potential of the enzyme for biodegradation of starch. Thus, the AMYT can be introduced as a potential candidate for industrial starch liquefaction processes.
Sequence data of this study have been deposited in the GenBank database with the submission ID OR193746.
The authors declare that they have no conflicting interest.
Not applicable
Not applicable
1.Tiwari SP, Srivastava R, Singh CS, Shukla K, Singh RK, et al. (2015) Amylases: an overview with special reference to alpha amylase. Journal of Global Biosciences 4: 1886-1901.
2.Nigam PS (2013) Microbial enzymes with special characteristics for biotechnological applications. Biomolecules 3: 597-611.
3.Sharma A, Gupta G, Ahmad T, Mansoor S, Kaur B (2021) Enzyme engineering: current trends and future perspectives. Food Reviews International 37: 121-154.
4.Ferrer M, Beloqui A, Timmis KN, Golyshin PN (2008) Metagenomics for mining new genetic resources of microbial communities. Microbial Physiology 16: 109-123.
5.Prayogo FA, Budiharjo A, Kusumaningrum HP, Wijanarka W, Suprihadi A, et al. (2020) Metagenomic applications in exploration and development of novel enzymes from nature: a review. Journal of Genetic Engineering and Biotechnology 18: 1-0.
6.Sharma P, Mondal K, Mondal KC, Thakur N (2022) Hunt for α-amylase from metagenome and strategies to improve its thermostability a systematic review. World Journal of Microbiology and Biotechnology 38: 203.
7.John J (2023) Biochemical and biotechnological aspects of microbial amylases. In Polysaccharide Degrading Biocatalysts 191-204.
8.Culligan EP, Sleator RD, Marchesi JR, Hill C (2014) Metagenomics and novel gene discovery: promise and potential for novel therapeutics. Virulence 5: 399-412.
9.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, et al. (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic acids research 31: 3784-3788.
10.Teufel F, Almagro Armenteros JJ, Johansen AR, Gíslason MH, Pihl SI, et al. (2022) SignalP 6.0 predicts all five types of signal peptides using protein language models. Nature biotechnology 40: 1023-1025.
11.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular systems biology 7: 539.
12.Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular biology and evolution 35: 1547.
13.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ
(2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nature protocols 10: 845-858.
14.Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, et al. (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic acids research 46: W296-303.
15.Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry 72: 248-254.
16.Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680- 685.
17.Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry 31: 426-428.
18.Jane?ek Š (2002) How many conserved sequence regions are there in the α-amylase family. Biologia 57: 29-41.
19.MacGregor EA, Jane?ek Š, Svensson B (2001) Relationship of sequence and structure to specificity in the α-amylase family of enzymes. Biochimica et Biophysica Acta (BBA)- Protein Structure and Molecular Enzymology 1546: 1-20.
20.Vester JK, Glaring MA, Stougaard P (2015) An exceptionally cold-adapted alpha-amylase from a metagenomic library of a cold and alkaline environment. Applied microbiology and biotechnology 99: 717-727.
21.Lo HF, Lin LL, Li CC, Hsu WH, Chang CT (2001) The N-terminal signal sequence and the last 98 amino acids are not essential for the secretion of Bacillus sp. TS-23 α-amylase in Escherichia coli. Current Microbiology 43:170-175.
21.Acer Ö, Bekler FM, Pirinççioglu H, Güven RG, Güven K (2016) Purification and characterization of thermostable and detergent-stable α-amylase from Anoxybacillus sp. AH1. Food Technology and Biotechnology 54: 70.
22.Afrisham S, Badoei-Dalfard A, Namaki-Shoushtari A, Karami Z (2016) Characterization of a thermostable, CaCl2-activated and raw-starch hydrolyzing alpha-amylase from Bacillus licheniformis AT70: Production under solid state fermentation by utilizing agricultural wastes. Journal of Molecular Catalysis B: Enzymatic 132: 98-106.
23.Murakami S, Nagasaki K, Nishimoto H, Shigematu R, Umesaki J, et al. (2008) Purification and characterization of five alkaline, thermotolerant, and maltotetraose-producing α-amylases from Bacillus halodurans MS-2-5, and production of recombinant enzymes in Escherichia coli. Enzyme and Microbial Technology 43: 321-328.
24.Dey TB, Banerjee R (2015) Purification, biochemical characterization and application of α-amylase produced by Aspergillus oryzae IFO-30103. Biocatalysis and Agricultural Biotechnology 4: 83-90.
25.Yang H, Liu L, Li J, Du G, Chen J (2011) Heterologous expression, biochemical characterization, and overproduction of alkaline α-amylase from Bacillus alcalophilus in Bacillus subtilis. Microbial cell factories 10: 1-9.
26.Far BE, Ahmadi Y, Khosroshahi AY, Dilmaghani A (2020) Microbial alpha-amylase production: progress, challenges and perspectives. Advanced Pharmaceutical Bulletin 10: 350.
27.Bhatt K, Lal S, Srinivasan R, Joshi B (2020) Molecular analysis of Bacillus velezensis KB 2216, purification and biochemical characterization of alpha-amylase. International Journal of Biological Macromolecules 164: 3332-3339.
28.Dey TB, Banerjee R (2015) Purification, biochemical characterization and application of α-amylase produced by Aspergillus oryzae IFO-30103. Biocatalysis and Agricultural Biotechnology 4: 83-90.
29.Liu X, Xu Y (2009) Molecular cloning and characterization of an α-amylase with raw starch digestibility from Bacillus sp. YX-1. Annals of microbiology 59: 91-96.
30.Afifi AF, Kamel EA, Khalil AA, Fawzi MF, Housery MM (2008) Purification and characterization of α-amylase from Penicillium olsonii under the effect of some antioxidant vitamins. Global Journal of Biotechnology and Biochemistry 3: 14-21.
31.Uma Maheswar Rao JL, Satyanarayana T (2007) Purification and characterization of a hyperthermostable and high maltogenic α-amylase of an extreme thermophile Geobacillus thermoleovorans. Applied biochemistry and biotechnology 142: 179-193.
32.Mehta D, Satyanarayana T (2013) Biochemical and molecular characterization of recombinant acidic and thermostable raw- starch hydrolysing α-amylase from an extreme thermophile Geobacillus thermoleovorans. Journal of Molecular Catalysis B: Enzymatic 85: 229-238.
33.Asgher M, Asad MJ, Rahman SU, Legge RL (2007) A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. Journal of food engineering 79: 950-955.
34.Allala F, Bouacem K, Boucherba N, Azzouz Z, Mechri S, et al. (2019) Purification, biochemical, and molecular characterization of a novel extracellular thermostable and
alkaline α-amylase from Tepidimonas fonticaldi strain HB23. International Journal of Biological Macromolecules 132: 558-574.
35.Priyadarshini S, Ray P (2019) Exploration of detergent-stable alkaline α-amylase AA7 from Bacillus sp strain SP-CH7 isolated from Chilika Lake. International journal of biological macromolecules 140: 825-832.
36.Arabaci N, Arikan B (2018) Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Preparative Biochemistry and Biotechnology 48: 419-426.
37.Wang X, Kan G, Ren X, Yu G, Shi C, et al. (2018) Molecular cloning and characterization of a novel α-amylase from Antarctic Sea ice bacterium Pseudoalteromonas sp. M175 and its primary application in detergent. BioMed research international 27: 2018.
38.Hmidet N, Jemil N, Nasri M (2019) Simultaneous production of alkaline amylase and biosurfactant by Bacillus methylotrophicus DCS1: application as detergent additive. Biodegradation 30: 247-258.
39.Lu Z, Wang Q, Jiang S, Zhang G, Ma Y (2016) Truncation of the unique N-terminal domain improved the thermos-stability and specific activity of alkaline α-amylase Amy703. Scientific reports 6: 22465.
40.Vester JK, Glaring MA, Stougaard P (2015) an exceptionally cold-adapted alpha-amylase from a metagenomic library of a cold and alkaline environment. Applied microbiology and biotechnology 99: 717-727.
41.Wu X, Wang Y, Chen X, Chen J (2018) Purification and biochemical characterization of a thermostable and acid- stable alpha-amylase from Bacillus licheniformis B4-423. International journal of biological macromolecules 109: 329-337.
42.Abd-Elaziz AM, Karam EA, Ghanem MM, Moharam ME, Kansoh AL (2020) Production of a novel α-amylase by Bacillus atrophaeus NRC1 isolated from honey: Purification and characterization. International journal of biological macromolecules 148: 292-301.
View PDF