Author(s): <p>Arjun Khanna*, Amit P Jose, Kartik Deshmukh, Dhruv Talwar, Satyam Agarwal and Deepak Talwar</p>
Interstitial lung diseases (ILDs) are a group of heterogenous disorders characterized by varying degree of fibrosis in lung [1]. Work-up for connective tissue diseases (CTDs) is an important component of evaluation of a patient with ILD. CTDs affect connective tissue in various organs resulting from poorly controlled autoimmune responses, and associated inflammation. Overall ILD has been documented in up-to 15% of patients with CTD, with rates as high as 26% in Systemic Sclerosis (SS) [2]. Lung involvement contributes to mortality in CTDs, and the management of ILDs depends on the presence and severity of underlying CTDs. Hence it is necessary to understand the clinical presentation of CTD-ILDs
Interstitial lung diseases (ILDs) are a group of heterogenous disorders characterized by varying degree of fibrosis in lung [1]. Work-up for connective tissue diseases (CTDs) is an important component of evaluation of a patient with ILD. CTDs affect connective tissue in various organs resulting from poorly controlled autoimmune responses, and associated inflammation. Overall ILD has been documented in up-to 15% of patients with CTD, with rates as high as 26% in Systemic Sclerosis (SS) [2]. Lung involvement contributes to mortality in CTDs, and the management of ILDs depends on the presence and severity of underlying CTDs. Hence it is necessary to understand the clinical presentation of CTD-ILDs
Most studies about clinical presentation of CTD-ILD have been performed in western population, affecting the generalizability to the Indian population. In the present study, we attempt to describe the clinico-radiological characteristics of patients with CTD-ILD in India. This is a retrospective study, and we report the data from CTDILD patients who presented at tertiary care center in Northern India.
We conducted a retrospective analysis of data collected from January 2019 - December 2019 at Metro Center for Respiratory Diseases, Noida, India. All patients in this study were seen in the referral practice of the primary investigator. The institutional ethics committee approved the study protocol and waived the requirement for informed consent of the subjects.
As regards diagnosis of CTDs, patients were diagnosed based on American College of Rheumatology/ European League against Rheumatism criteria [3-5]. ILD was confirmed based interpretation of HRCT by an experienced radiologist. All patients were discussed in a multidisciplinary team meeting, and expertise was sought from other physicians on a case-by-case basis. We excluded patients with a known history or on active treatment of tuberculosis and sarcoidosis.
The patients were also given a questionnaire to capture the demographic variables, presenting symptoms (symptoms that prompted visit to physician in the first place) and self-reported duration of symptoms. As regards initial presenting symptoms, they were classified as “;CTD- dominant”; if they had symptoms such as fever, fatigue, joint aches, muscle pain, rash , swelling, etc. Similarly patients were classified as having “;ILD-dominant”; symptoms if they presented with cough, chest pain, sputum production and shortness of breath.
Clinical data including physical examination, diagnosis of CTD and ILD, radiology and serology(auto antibodies) were ascertained from the chart and health records of the patients. We also captured the relevant medication history (excluding the exhaustive treatments for co-morbidities) and history of usage of oxygen therapy for the patient at the time of presentation to our center Data on lung biopsy, presence/absence of PAH, coexisting co-morbidities and drug history was also captured from medical records. We did not subject the patients to cardiac catheterization to confirm diagnosis of every PAH patient included in the study. The pulmonary arterial pressure was measured using the echocardiography. A value greater than or equal to 35 mm Hg is considered PAH and classified as follows: mild PAH (35-50 mm Hg), moderate PAH (50-70 mm Hg), and severe pulmonary hypertension (> 70 mm Hg)
Patients underwent pulmonary function testing at the time of initial visit according to ATS/ERS criteria for measurement of spirometry, lung volumes and DLco Spirometry and HRCT were performed initially and then at an interval of 6 - 12 months; the decision to perform these tests left to the discretion of the treating physician. HRCT scans and PFT results included in the analysis were conducted at the time of presentation to the center [6, 7].
The data was analysed using IBM SPSS 20.0. Numbers and percentages were provided for dichotomous and polychotomous variables, and means and standard deviations were calculated for continuous variables. Changes in FVC and DLco were measured across CTD subtypes - Sjogren, progressive systemic sclerosis (PSS), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), mixed connective tissue disease (MCTD), and KruskalWallis test was used to test the significance of the findings.
A total of 50 patients were included in the study, with 33(78%) of the patients being females. Among females, the mean age was 58 years, with majority having attained menopause and the median age of menopause being 44 years.
Baseline demographic characteristics, clinical characteristics, pulmonary function testing results and medication history are summarized in Table 1.
| Total N = 50 | |
| Mean age at presentation (±SD) | 59± 11.5 yrs |
| Mean duration of symptoms before diagnosis |
2.86 ± 1.9 years |
| Mean BMI (±SD) | 25.662 ± 3.1 kg/m2 |
| Ever-smokers | 7(14%) |
| Co-morbidities (%) -Hypothyroidism -Hypertension -Diabetes Mellitus (DM) -Osteopenia -Coronary artery disease(CAD) -Anemia -GERD |
14(28%) 21(42%) 6(12%) 15 (30%) 8(16%) 29(58%) 17(34%) |
| Respiratory Symptoms -Cough -Shortness of breath - Acute Rhinosinusitis |
42 (84%) 49 (98%) 26 (52%) |
| Pulmonary Function tests -Mean FEV1/FVC -Mean FVC(% pred) -Mean DLco(% pred) |
80.82% ± 12.27% 62.59% ± 16.46 % 48.5% ±17.12%. |
| Medication history -LABA -SABA -Steroid usage -Mycophenolate mofetil -Azathioprine |
40(80%) 48(96%) 49(98%) 31(62%) 13(26%) |
| Oxygen therapy | 23(46%) |
At the time of initial presentation 98% of the patients were tachypneic and hypoxic (mean PaO2 of 75 mmHg) at presentation.
Classical Velcro crackles were heard on auscultation in all patients with 92% (n=46) of subjects having bilateral basal crackles, and wheeze present in 48% (n=24) patient. 14 % (n=7) patients had clubbing. As regards initial presentation, 68% of patients presented with CTD-dominant symptoms to begin with; rest presented with ILD- dominant symptoms.
Overall, 48% (n=24) reported to have no PAH. The distribution of PAH across CTDs in mentioned in figure 1.
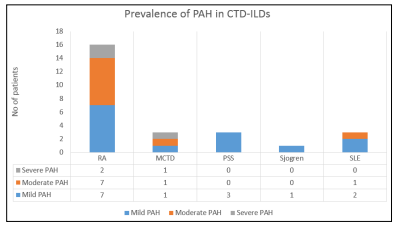
Figure 1: Distribution of PAH across CTDs
Overall, the most common auto-antibody detected was Anti-RF (mean value of 81.7 IU/mL), occurring in about 56% of total patients included in our study
Anti-CCP was detected in 38% (12/31) with RA. Both anti-RF and anti-CCP positivity was seen in 29% (9/31) of RA patients. AntiU1RNP was detected in all patients with MCTD. ANA was seen in 80% (4/5) of patients with ANA, 100% (5/5) of patients with PSS, 83% (5/6) of patients with MCTD. All SLE patients were positive for anti dsDNA and 33% (1/3) for Anti Smith antibody. In PSS, 20% (1/5) had anti-centromere antibodies and 80% (4/5) had anti SCL-70. Anti SSA/SSB were detected in 19%, 80% and 33% of patients with RA, SS and SLE respectively. Combined SS-A/SS-B positivity was seen in 20% (1) of patients with Sjogren.
Confirmatory biopsy results were available for 8 patients (16%). Lung biopsy revelated usual interstitial pneumonia (UIP) in 3, nonspecific interstitial pneumonia (NSIP) in 2, organizing pneumonia (OP) in 2, and was unclassifiable in 1 patient.
Overall, most common pattern on HRCT was UIP with 46% (n=23), followed by NSIP 36%(n=18). The distribution of CT patters across CTDs in evaluated patients is mentioned in table 2.
| RA (n = 31) | 12 | 13 | 1 | 5 |
| MCTD (n = 6) | 4 | 1 | 1 | 0 |
| PSS (n = 5) | 4 | 1 | 0 | 0 |
| Sjogren (n = 5) | 2 | 2 | 1 | 0 |
| SLE (n=3) | 1 | 1 | 1 | 0 |
Mean Change in FVC and mean change in DLco of the patients over the period of 6-12 months were -0.0926 L±0.18L and 0.16% respectively (both statistically non-significant)
Our study is the first single center study conducted in India that intends to capture the clinical presentation of patients with CTD-ILD. The results from our study adds to the data previously captured through India ILD registry [8].
The mean age of our sample is similar to ones reported previously in literature [8-11]. Majority (78%) of patients in our study were females - this is consistent with the percentage of females in the CTD-ILD subgroup (n = 151, F = 111, M = 40) in India ILD registry [8].
RA-ILD was the most common sub-type CTD-ILD in our study. Other studies conducted in India and other countries have reported similar findings [12]. The male female ratio in patients with RAILD was 1:2.4 indicating a clear female dominance. This is in contrast to previous studies which associated male sex with an increased risk of RA-ILD [13, 14]. A study in UK has shown almost equal gender distribution of RA-ILD in males and females; a few others have shown female preponderance, highlighting the epidemiological variations on a local scale.
ILD is sometimes the initial manifestation of CTD, and 32% of patients in our study had predominant respiratory complaints in their initial presentation( pulmonary symptoms as initial manifestation) [15, 16]. This is in contrast to studies conducted in China and Sri-Lanka that reported much lesser percentage of patients presenting with pulmonary symptoms in the first place [10, 17]. This observation is important since patients without specific rheumatological symptoms and/or antibodies in the early stage can be mis-diagnosed in the initial presentation. We cant explain the higher percentage of patients presenting with pulmonary symptoms as initial manifestation in our study; we believe that referral patterns to our center, which is an tertiary care pulmonology center, might have played a role.
Our study reveals that the predominant HRCT pattern in CTD-ILD was UIP (23/50) followed by NSIP (18/50). This contrasts with the multiple previous studies which report NSIP as the predominant HRCT pattern in CTD-ILDs [18]. Another interesting aspect to note is the distribution of NSIP/UIP in RA-ILD and PSS-ILD that conflicts with previous data - we noticed greater prevalence of NSIP in RA-ILD and lower prevalence of NSIP in PSS [19]. NSIP has been observed in up-to 43.75% of patients with RAILD, and the small sample size in our study might have led to increased prevalence of NSIP in RA-ILD [20]. Increased age has been associated with UIP in PSS; considering the mean age of 59 yrs in our study, this might have led to predominance of UIP in PSS-ILD [21].
The prevalence of GERD and CAD observed in our study is similar to previously reported data on co-morbidities in CTD-ILD [22-24]. Osteopenia was observed in 30% of total study group. High prevalence of osteoporosis and osteopenia among newly diagnosed ILD patients were reported by Alhamad et al, and these findings highlight early screening and aggressive management for osteopenia in patients with CTD- ILD [25]. An interesting finding was presence of hypothyroidism in 28% patients. Oldham et al has demonstrated prevalence of hypothyroidism with Interstitial lung disease mainly IPF, that with more in females as compared to males [26]. The clinical significance of hypothyroidism in CTD-ILD has not been studied in detail, and warrants further evidence generation.
52% of patients in our study were reported to have PAH. Most reliable studies on PAH in CTD-ILD have been conducted in PSS, and not surprisingly PSS-ILD accounts for ~75% of CTD-PAH cases [27]. The results from our study further reinforces the need for regular PAH screening in patients with CTD-ILD. The patterns of auto-antibodies is similar to the patters reported in previous studies [28].
Most of the CTD ILD patients were seen to have restrictive defect on pulmonary function as reported in literature [9, 29]. The mean change in FVC and DLco over a period of 6-12 months was insignificant. The stability of lung function indicates that the disease was milder in the sample than in historical samples of patients with IPF [30].
The present study was a single center study, affecting the generalizability of the findings from the study. We included retrospective data of patients in the study which could be affected by selection bias and referral patterns
RA-ILD is the most common CTD-ILD subtype identified at our center. We reported UIP as a dominant pattern in patients with CTD-ILD ( especially in patients with PSS-ILD) which is in contrast to studies conducted in western population.
We recommend further prospective registries to explore the clinical, radiological and prognostic characteristics of CTD-ILD in India.
