Author(s): Gaosi Xu
Diabetes mellitus is a growing burden on global healthcare. Although the definition of Diabetic Kidney Disease (DKD) is that the kidney disease due to type 1 or 2 diabetes, the clinical diagnosis of DKD remains controversial. The diagnosis of DKD is highly subjective, depending on the doctor’s judgment and experience. It is necessary to distinguish the non-diabetic kidney disease from DKD at an early time, however, it is also seems difficult and inefficient. Up to date, there is lack of the diagnostic flow diagram for the disease. In this mini-review, we for the first time focused to create a diagnostic workflow for the disease
Diabetes mellitus (DM) was estimated 451 million people worldwide in 2017, and the number is expected to increase to 693 million by 2045 [1]. The prevalence of DM has increased by about 10-fold during the past 3 decades in China, which can be attributed to the rapid industrialization and urbanization, increased consumption of high-calorie diet, sedentary lifestyle, and rapid growth of the older population [2]. It is estimated that about 40% of patients with DM will develop diabetic kidney disease (DKD), which was a major cause of morbidity and mortality in diabetes patients and the leading cause of end-stage renal disease (ESRD) in the world [1, 2]. DKD has also become the main cause of chronic kidney disease (CKD) [3]. Even in its early stages, the risk for cardiovascular events is significantly increased [4].
DKD was defined clinically by persistently high urinary albuminto-creatinine ratio (ACR) > 30 mg/g and/or sustained reduction in estimated glomerular filtration rate (eGFR) below 60 ml/ min per 1.73 m 2 [5]. The Consensus Conference on Chronic Kidney Disease and Diabetes convened by the American Diabetes Association (ADA) in collaboration with the American Society of Nephrology (ASN) and the National Kidney Foundation (NKF) recommended that identifying and monitoring DKD relies upon the assessments of kidney function usually with eGFR and kidney damage usually by estimation of ACR [6]. However, neither albuminuria nor GFR decline are DKD-specific markers, thus the diagnosis of DKD is highly subjective and greatly depends on assumptions. Confirmation of albuminuria or low eGFR requires two abnormal measurements at least 3 months apart. In addition, numerous studies have shown that the treatment of hypertension, no matter what drugs are used, has a beneficial effect on proteinuria. In addition, clinicians must be highly suspicious of detecting RAS in diabetic patients, especially those who have established coronary and/or peripheral atherosclerotic disease and compromised renal function.
The clinical stages of DKD were considered to begin from early glomerular hyperfiltration, followed by the development of microalbuminuria, macroalbuminuria, and then declined GFR However, in recent studies of type 2 DM, many patients with DKD do not manifest the above mentioned classic stepby-step progression [5, 6]. On the other hand, when persistent microalbuminuria becomes detectable, DKD has already progressed to the stage 3 CKD, therefore, the onset of DKD is difficult to detect. In addition, there is now a growing evidence to suggest that many patients with type 1 or 2 diabetes can follow a nonalbuminuric period to renal function loss [7, 8].
DKD can occur either alone or superimposed on non-diabetic kidney disease (NDKD). Although the differential diagnosis of DKD and NDKD has been explored in numerous previous studies, the effective, safe, and scientific identification of NDKD from DKD remains a major challenge. Consequently, there is an urgent need for clinicians to develop a diagnostic flow diagram for distinguishing diabetic patients with NDKD from DKD. If renal biopsies were feasible in all patients without safety considerations, many patients would probably be diagnosed with early stages of DKD. Although biopsy is the gold standard for diagnosing DKD, the procedure is rarely used in routine clinical practice in uncomplicated cases due to its invasive nature, and is often reserved for cases with severe albuminuria, a fast decline in GFR, or where differential diagnoses are required.
DKD, also termed CKD due to diabetes, is defined in both type 1 and type 2 diabetes with concurrent presence of diabetic retinopathy (DR) and absence of signs of other forms of renal disease [9]. DR is a common microvascular complication of T2DM and shared pathophysiological mechanism with DKD. It is generally believed that in patients with T2DM, the occurrence of DR is often accompanied by the development of DKD, and that patients who lack DR are likely to have NDKD [10]. DKD and DR are considered as interrelated diabetic vascular complications since kidneys and retina share similar size arteries. However, the absence of DR was a significant predictor for the development of NDKD but was not an exclusion criterion for DKD. It is quite difficult to differentiate DKD from other glomerular diseases if DR is absent in patients, and the only way to do this is to perform renal biopsies [11].
Most recently, a meta-analysis which included up-to-date the largest sample size studied of 4990 patients with diabetes and biopsy-proven DKD, found that if the test for DR is positive, the probability of DKD increased only to 42%, but if the test for DR is negative, the probability of DKD would decrease to 10% [12]. In addition, although the mean sensitivity was 0.25 (95% CI 0.16 to 0.35), the mean specificity of proliferative DR for detection of DKD was 0.98 (95% CI 0.92 to 1.00, Figure 1). 95% of patients with T1DM and DKD have DR, so the absence of retinopathy has been accepted as an important clinical indication for renal biopsy in T1DM and T2DM patients with renal disease [13, 14].
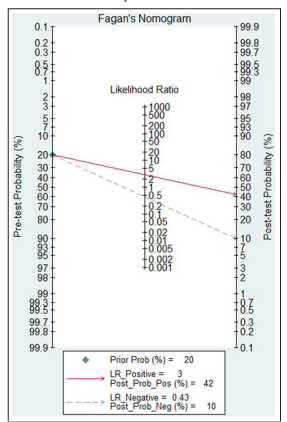
Figure 1: Fagan nomogram of diabetic retinopathy for the diagnosis of diabetic kidney disease [12]
Recent extensive studies suggest that the incidence of NDKD, such as IgA nephropathy and membranous nephropathy in patients with T2DM varies from 27% to 82.9% [15, 16]. Totally, there were 16 types of NDKD, including primary and secondary glomerulonephritis [17]. The most common form of primary glomerulonephritis in diabetes was idiopathic membranous nephropathy, which accounted for 46% of the cases, followed by IgA nephropathy and focal segmental glomerular sclerosis [13]. Atypical features for NDKD include sudden onset of low eGFR or rapidly decreased eGFR, abrupt increasing in albuminuria or progression of nephrotic or nephritic syndrome, refractory hypertension, signs or symptoms of another systemic disease, and more than 30% eGFR decrease within 2-3 months of initiation of a renin-angiotensin system inhibitor [5]. The National Kidney Foundation Kidney Disease: Outcomes Quality Initiative (KDOQI) working group for diabetes and CKD suggested that the absence of DR, fast deterioration of GFR, rapidly increased or nephrotic-range albuminuria (>2500 mg/g), active urinary sediments, refractory hypertension, or signs or symptoms of other systemic diseases should raise the suspicion of NDKD [5].
Screening for DKD should be performed annually for patients with T1DM beginning 5 years after the diagnosis and annually for all patients with T2DM beginning at the time of diagnosis [18].The presumptive diagnostic flow diagram for DKD was created for the first time and demonstrated in Figure 2. However, in the future, the international large sample, multi-centered randomized clinical trials should be performed to verify the precision and feasibility of the current workflow.
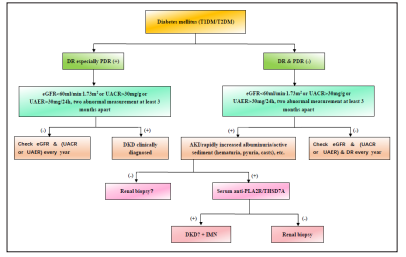
Figure 2: Patients with type 1 or 2 diabetes should be examined for diabetic retinopathy (DR) especially proliferative diabetic retinopathy (PDR). When the test for DR especially PDR is positive, diabetic kidney disease (DKD) clinically diagnosed if eGFR < 60ml/min.1.73m2 or UACR > 30mg/g or UAER > 30mg/24h is two abnormal measurements at least 3 months apart, but if not, the patients should check eGFR or UACR or UAER every year. When the test for DR and PDR are negative, the patients should take the test for acute kidney injury (AKI)/ rapidly increased albuminuria/active sediment (hematuria, pyuria, casts) if eGFR < 60ml/min.1.73m2 or UACR > 30mg/g or UAER > 30mg/24h is two abnormal measurements at least 3 months apart, but if not, the patients should check eGFR and (UACR or UAER) and DR every year. When the tests for AKI/rapidly increased albuminuria/active sediment (hematuria, pyuria, casts) are negative, renal biopsies should be considered to differentiate DKD from other kidney diseases. However, when the test for AKI/ rapidly increased albuminuria/active sediment (hematuria, pyuria, casts) is positive, renal biopsies should be performed if serum antiPLA2R/THSD7A are positive, but if not, idiopathic membranous nephropathy clinically diagnosed with suspected DKD.
Ethics Approval and Consent to Participate: Not applicable
Consent for Publication: Not applicable
Availability of Data and Materials: Not applicable
This work was supported by the National Natural Science Foundation of China (No. 81970583) and the Nature Science Foundation of Jiangxi Province (No. 20181BAB205016).
QW performed the literature search, reviewed articles wrote the manuscript. GX reviewed the articles and provided secondary reviews during the manuscript preparation. All authors have read and approved this information before submission.
