Author(s): Kafor Bernard*, Nnadi Godfrey, Amadi Darlington, Nweke Ikechukwu and Agu Vincent
Since the middle of the 20th century, the cervical cancer screening test has been a widely used method for identifying precursor cells in cancer cases. The disease may be eradicated if premalignant cells are found early enough. According to studies, pap smear-based screening programs for cervical cancer can dramatically reduce the number of fatalities worldwide attributable to cervical cancer. Understanding the female reproductive system is crucial for the efficient and representative collection of cervical samples, and a methodical and cautious approach to sample collection is required. Following directions and using the appropriate instruments for collecting specimens are crucial for producing high-quality results. In order to help providers better comprehend the subject, the work sheds light on the procedure and method of cervical sample collection.
The World Health Organization (WHO) reports that cervical cancer is the second most frequent malignancy among women, accounting for about 300,000 deaths worldwide each year [1]. Developing nations account for 86% of these fatalities. The primary cause of this disparity is the lack of structured screening programs which utilizes the Papanicolaou test (Pap test), created by Dr. Georges Papanicolaou in the 1940s, in these poorer nations [2].
The Papanicolaou smear (Pap test) is a common screening test for uterine cervix cancer, which was first reported in 1928, and by 1941, its effectiveness had been established. This test has since gained widespread acceptance as a clinical tool for cancer early detection [3]. Much of the work done in the development of this test commonly identified as “pap test” has been credited to Dr. G.N. Papanicolaou.
This cancer is completely avoidable and curable because the progression from the precursory stage to malignancy is gradual and previous report has shown that no other test has proved as effective in eradicating cervical cancer through the identification of curable precursor lesions as pap test [4]. The Papanicolaou smear can indeed identify precancerous alterations in cells that are not evident to the naked eye. Regular screening, detection, diagnosis, and therapy of precursor lesions open a wide window of opportunity for the prevention of invasive malignancies and fatalities associated with them.
It has been demonstrated that coordinated cervical cancer screening programs using pap tests significantly lower cervical cancer incidence and cancer-related deaths globally [5]. Women in less developed countries, like Nigeria, typically have access to this test through population screening, which involves setting up camps in urban and rural areas, or by opportunistic screening. The conventional or liquid-based Pap test has withstood the test of time.
The best way to identify anomalies in the cervical epithelium that precede invasive cancer is still a routinely obtained smear from the Squamo columnar Junction (SCJ), properly fixed, and well stained. The conventional or liquid-based Pap test has however withstood the test of time. Following collection, these abnormalities can be confirmed by using guided biopsy such as colonoscopy. These abnormalities can subsequently be ablated using either cryotherapy or a broad loop excision of the transitional zone (TZ) during a loop electrosurgical excision technique. Despite the fact that the Pap smear has proven useful for decades, one of the technical difficulties that operators frequently encounter is gathering a representative sample for the inquiry. Health professionals involved in this process need to be sufficiently exposed to the appropriate methods and tools employed in order to surmount this barrier. Surmounting this barrier is indeed the objective of this study.
The female reproductive system is composed of internal and external genitalia. The vulva and its supporting components make up the external genitalia. The uterine tubes, uterus, and vagina make up a three-part system of ducts that make up the internal genitalia. The ovaries which are the body’s main reproductive organs, are connected to this system of ducts (see figure 1). The Mullerian (paramesonephric) ducts are two embryonic structures that develop into the uterus, the fallopian tubes, and the top two thirds of the vagina. The uterus and proximal vagina are formed by the caudal fusion of the two Mullerian ducts. However, the top portion is still divided into the fallopian tubes. The creation of the vaginal and uterine septa is the result of imperfect duct fusion. The lower vagina and vestibule are formed by the fused ducts contacting the urogenital sinus.

Figure 1: Female Internal Genital Tract
The fibromuscular organ known as the cervix connects the vagina and the uterine cavity. The cervix measures around 4 cm in length and 3 cm in width. A parous woman’s cervix is significantly larger than a nulliparous woman’s, and that at reproductive age is significantly larger than a postmenopausal woman’s [6]. The cervix is located both internally and externally. The ectocervix, also known as the portio vaginalis, is the exterior surface embedded within the vagina and is discernible during vaginal inspection. Stratified squamous epithelium covers it. The remainder is contiguous with the uterus’s main body. Ectocervix, endocervix (the canal within the cervix), external os (the entrance of the endocervix in the vagina), and internal os (the opening of the endocervix within the uterus) are the different components of the cervix. A straightforward columnar glandular epithelium lines the endocervix. One anterior, two lateral, and one posterior fornice are formed as a result of the cervix’s extension into the vagina. Exfoliated cells frequently gather in these fornices’ secretions. Deep mucosal infoldings known as plicae palmatae ascend to the stroma inside the endocervix, where they produce crypts that resemble branching glands. The canal is small, usually only 2-3 mm, and frequently contains a mucus plug whose morphological characteristics vary depending on when ovulation occurs.
The vagina extends from the vestibule of the vulva, and on either side of the vaginal introitus are mucin-producing glands, including Bartholin’s glands, which are located in the lower vaginal wall and provide both lubrication and protection. The vaginal epithelial lining is covered by non-keratinizing squamous epithelium. It is sensitive to hormones, going through cyclical changes when in the reproductive age range, and atrophies with menopause.
The vulva is external portal of the female genital tract. It consists of labia majora and minora, which are both squamous keratinizing epithelium-covered. The labia majora’s inner surface has numerous sebaceous and apocrine glands, whereas the outside surface has hair. Sebaceous glands are located on the labia minora’s outer surfaces, whereas the inner surfaces blend into the vagina. When a person reaches adolescence, the pigmentation of their labia begins to fade, leaving only a thin layer of keratin on the inner surface of the labia minora. The vestibule is covered by this epithelium all the way to the hymen. The clitoris, the female equivalent of the penis, is situated anteriorly between the labia minora and is equipped with a retractile, prepuce-like structure. The opening of the urethra, the final segment of the urinary tube, is about 1 cm behind the clitoris. The inguinal lymph nodes, which serve as the principal location of metastases in malignant tumors of the vulva, get lymphatic drainage from the vulva.
The squamolunar junction is an important landmark in cervical pathology. Simply put, it is a junction of the endocervical glandular epithelium and the ectocervical squamous epithelium. The two types of epithelium, at birth are locate at the os of the cervix and changes positions at puberty. The SCJ develops as a result of a continuous remodeling process brought on by uterine growth, cervical growth, and hormonal status, according to Dash et al [7]. The original SCJ everts and a sizeable portion of the columnar epithelium migrate to the ectocervix during this phase and this is known as the ectropion. The changes as result of ectropion or eversion results in the formation of new vulnerable part of the cervix known as the transformation zone (TZ). View FIGURE 2. Transformation is known to be area of active metaplastic changes, from glandular epithelium to a more robust squamous epithelium due to recurrent infections and repeated stress from parturition [6,8].
The vulnerability of the TZ also makes it prone to human papillomavirus which is the major etiological agent in the development of cervical cancer. According to Dash et al. over 90% of cervical malignancies have their root from the TZ, and hence the collection cells for pre-invasive evaluation is centered on this very zone. Metaplastically altered glands with blocked orifices are prone to mucus distention, which can even make them macroscopically evident as rounded nodules on the ectocervix known as nabothian follicles. The cervix contracts during menopause, and the SCJ moves higher up into the endocervical canal. The appropriate sampling of the TZ and SCJ, quick wet fixation, and staining of the smear are crucial for the accuracy of cervical cytology in the diagnosis of precancerous lesions.
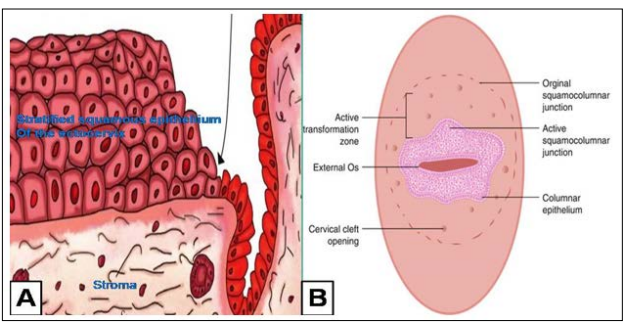
Figure 2: Diagrams Depicting Squamocolumnar Junction and the Associated Landmarks. A. Ectocervix (left of arrow) and Endocervix (right of arrow). Squamocolumnar Junction (arrow head). B. The Macroscopic View of the Cervix.
The most typical and well accepted technique for collecting cells for cytodiagnosis is cervical scraping. The final results of this procedure depend not only on the skill of the cytologist doing the analysis, but also on the technique utilized to gather the samples. Cells are gathered from the entire SCJ and the nearby TZ in this. Two samples—one from the endocervix and one from the ectocervix—are collected and spread out on microscopic slides as part of the conventional cervical scraping method [9].
There are other techniques for the collection of cervical cells diagnosis, including
Cervical cytology sampling, involves a variety of sample collection devices, and prominent among them are spatula, endocervical brush and the speculum (see figure 3). The first two picks the target sample cells, while the last creates to the cervix through the vaginal canal. Ayre spatula and Aylesbury (with extended tips) spatula are the two main types of spatulas used in conventional Pap smears, and both produce similar diagnostic outcomes. Endocervical brushes include the cytobrush, which has a narrow tip and soft bristles that are easy to insert into the endocervical canal, and the cervex, which is a broom-like device made of flexible plastic filaments. The lateral bristles of the broom are bent against the ectocervix while the central bristles are inserted into the endocervical canal in the case of the cervex. Cervex brush is commonly used in the LBC. According to Abyn et al [10]. More endocervical specimen are collected when spatula and cytobrush are used for the same patient, than when either of the two is used separately. Ayre spatula is common used in lower-income nations like Nigeria. Cotton tipped swab is an ineffective tool for cervical sample collection as they rarely identify pre cancerous cells.11,10 Samples for Pap smears are typically collected with Cusco’s speculum, also referred to as the bivalve speculum. We have two different kinds of speculum: metallic and plastic. The former is made of stainless steel and is reusable, whilst the latter is made of plastic.
Other requirements for the cervical sample collection might include specimen sample container with fixatives and glass slides (see figure 3). Spray fixatives are mostly used for samples that will be transported to a distant laboratory.

Figure 3: Major Devices used for Cervical Sample Collection.
A) Spatula(Ayre & B) cytobrush C) Detachable Head Cytobrush-Commonly for Liquid Base Cytology(LBC). D) Metal Speculum (left); Plastic Speculum Right. E) Slide & Specimen Container with Fixative.
Patients Preparation and Procedure for Sample Collection The Pap smear should be collected two weeks following the first day of the last menstrual cycle. The patient needs to be told not to use douches, spermicides, or vaginal medicines 48 hours before the collection. Additionally, for 24 hours previous to collection, the patient should abstain from sexual activity. It is not advised to take a cervical specimen from a patient who has douched or is menstruating. It is not advisable to use lubricants for sample collection, as they might render them unsatisfactory. Warm water is suggested, but if lubricant must be used to alleviate patient discomfort, water based lubricant such as KY jelly can be allowed.
The sociodemographic information such as age, parity, last childbirth, biopsy/cytology previous history, date of last menstrual period, history of contraceptives use, exact anatomical site of collection and equipment used, are requested from the patients at the collection/accession.
To ensure adequate sample collection for effective detection of precancerous and cancerous cells, the samples has to be collected from the transformation zone and the following steps observed
The second most prevalent kind of cancer among women is cervical cancer. Adopting a systematic screening program will help lower the disease’s incidence and deaths due to it. Using a brush or spatula/both, a qualified healthcare professional should be able to collect the sample correctly for the Pap smear slide. The material is then fixed, stained, and examined after being smeared onto a microscope slide.
