Author(s): <p>Gilson Gilmar Holzschuh*, Jorge André Ribas Moraes, Sérgio Boscato Garcia, Izete Zanesco, Liane Mahlmann Kipper and Rosana de Cassia de Souza Schneider</p>
This research presents a methodology for the recycling process via casting, in which aluminum cans and primary aluminum are transformed into a laminated tape, with the possibility of industrial application. Its objective was to incorporate materials of different properties that could favor the making of a laminated tape. The recycled aluminum was melted and then Cu and Mg was incorporated. For the design of the laminated tape, a cast was made to receive the molten aluminum. The aluminum was cast into this mold and three tapes were produced, with 2, 3 and 4 mm thickness. From these tapes, lamination process was applied to reduce their thickness to values close to 0.5 mm. The casting process of aluminum cans with the addition of commercial aluminum, plus the incorporation of copper and magnesium, demonstrated facilities for thickness reduction in the process of making laminated tapes and an elemental composition suitable to electrical conductivity application. After four lamination passes there was a reduction of 85.4% of the initial thickness, reaching (0.59 ± 0.04) mm of final thickness. The electrical conductivity was 45.96 to 47.67 IACS with a reduction of only 1.82 % after lamination. Therefore, a tape with electrical conductivity and 0.59 mm thickness was obtained from recycled beverage cans aluminum. In addition, the characterization presented for the 3 samples will allow the study of applications for these alloys and respective electrically conductive tapes.
Recycling is an economically and environmentally friendly process, because it prevents that the wastes are disposed of in landfills or in improper places, thus contaminating the environment [1]. With recycling, the volume of these wastes is reduced, as well as it avoids the amount of new raw material used in certain processes [2]. With each ton of recycled aluminum (Al), the extractions of 4 tons of bauxite are not needed. This saves up to 95 % in energy production, which corresponds to approximately 700 kg of crude oil [3].
When mentioning the term recycling, Al is the first to come to mind because it is a material that can be recycled countless times without losing its physical / chemical properties [4]. Some of the benefits of aluminum recycling go beyond the reduction of consumption of the energy and raw materials. Aluminum recycling involves social issues, since recycling is a source of income for many families of waste pickers, therefore, keeping people with a low education level in the labor market. Thus, resources are generated for application in local economies, developing the market at local and national levels [5,6]. According to Ghisellini and Ulgiati, in Italy, recycling has become the preferred practice in most organizations involved throughout the supply chain, from post-consumer waste collection to secondary raw material recovery, recycling and production. Al recycling in Brazil has been growing in recent years. In 2002, the index was 87 %, reaching 96 % in 2005 [7]. According to the Brazilian Aluminum Association -ABAL, almost all Al cans from beverages sold in 2017 returned to the production cycle, reaching an index of 97.3 %. Of the 303,900 tons of cans distributed in the Brazilian market in 2017; 295.8 thousand tons were recycled. This makes it an excellent choice, especially for carbonated beverage packaging (e.g., soda and beer). The recycling process has one of its biggest advantages in saving energy, since it uses only 5 % of the energy needed to produce the primary metal from ore [8]. There are others environmental advantages by recycled Al use, as and reduction of 95 % of air pollution and greenhouse gas emissions and 97 % of water use when compared to primary Al production [3].
Explored different compositions of recycled Al [9]. Recycled with primary Al, rice husk ash, magnesium and copper were melted through the gravity casting process and drained into a mold. Ashes from rice husk, magnesium and copper were added to the ingot to produce a metallic matrix that was characterized by several techniques. The inclusion of rice husk ash into molten aluminum was analyzed using scanning electron microscopy, density analysis, and Brinell hardness and Charpy impact force testing. The metallic matrix formed by the methodology that involved varying the addition parameters and metal incorporation appeared feasible for several industrial applications.
Aluminum has corrosion resistance, thermal and electrical conductivity, and is very ductile [10]. All these features make it suitable for the manufacture of very thin laminates, packaging, beverage cans, chemical industry containers, cables and electrical conductors. An Al electric conductor wire, in the proper proportion of the conductor wire area, can conduct as much electrical current as a copper conductor wire, which is twice more heavy and more expensive. For this reason, aluminum is widely used by the wire and cable industry [10]. However, primary aluminum has low resistance to mechanical stress and low levels of hardness. Therefore, for parts that are subject to high stress, aluminum resistance is not adequate [11]. There are several ways to improve the strength properties of a metal, chemical elements can be added and an alloy can be formed or by mechanical processes application, such as rolling or pressing [12]. Improved results can also be achieved by the application of heat treatment processes [13]. Mansurov et al. state that the relatively low value of casting technology provides a greater share of the demand for aluminum alloys [14]. This is besides aluminum’s corrosion resistance properties and excellent recyclability. Thus, the objective of this research was to perform the casting of Al cans, to form an alloy with the addition of commercial Al in the proportion of 50 %, and subsequent additions of copper (Cu) and magnesium (Mg), in order to develop a conductive laminated tape.
Recycled aluminum samples (beverage cans) were obtained through the Santa Cruz do Sul Waste Pickers Cooperative - RS - Brazil. To remove the moisture, the materials were inserted into a muffle furnace, with the temperature ranging of 150 - 200 °C. The materials were melted without separation of the can components in an industrial Grion oven in a steel crucible. The aluminum melting temperature was 750 °C, based on Verran and Kurzawa and after preparation for casting, the material was compacted by pressure to improve the melting process [15]. The stages of the casting process are described in Figure 1.
The first stage involved the Al cans casting and the formation of the 1st ingot. During the casting process, slag formed on the surface of the melt was removed for every 5 kg of cans introduced into the oven. At this stage of the research, 20 kg of recycled aluminum cans were used.
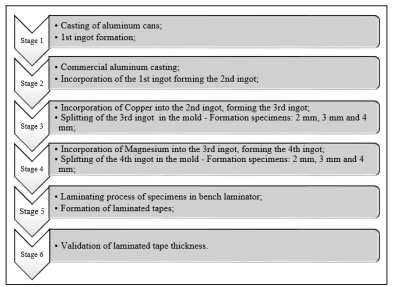
Figure 1: Methodological sequence of the casting processWith the mixture finally homogenized, the melt was poured into the mold. The melt, while still in a liquid state, was poured in small quantities into the mold for when solidified, would not become too thick.
After that, to commercial Al casting and incorporation of the 1st ingot to form the 2nd ingot. This step occurred through the casting of 10 kg of commercial Al classified as alloy 6063 T6. When the material was in a liquid state, the ingots of the first casting. At the end of the homogenization of the mixtures, the material was again poured into a mold and converted into an ingot 50:50 (50 % recycled cans and 50 % commercial Al alloy). This ingot was used to create a 0.5 mm thick laminated tape. The ingot formed presented 16 mm thick and was molded.
Cooper was incorporated into the 2nd ingot and it was obtained the 3rd ingot. In order to cast the 2nd and the 3rd ingot and making the laminated tapes, it was used a mold with suitable spaces to receive the melt (Figure 2).
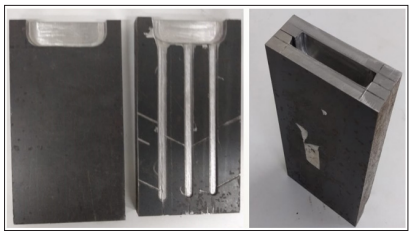
Figure 2: Chill mold made for pouring of the 2nd ingot
The mold preparation, a flat steel bar, SAE 1045, 30 cm long and 10 cm wide was used. This bar was cut in half to use one piece as a lid and the other piece was reamed with an 8 mm tool to form the channels. In the upper part of the mold, a cavity was made to prevent Al spillage from the second casting, when it was poured.
The pouring method wasby gravity casting with three channels of 2, 3 and 4 mm of depth. Thus, the specimens had their measurements described as shown in Table 1 Gas exhaustion care was according to Holzschuh et al. [9]. Samples were taken for chemical analysis, micrograph, density, impact and hardness testing to validate the mixture and verify properties.
| Test Specimen 01 | Test Specimen 02 | Test Specimen 03 | |
|---|---|---|---|
| Width (mm) | 08 | 08 | 08 |
| Length (mm) | 15 | 15 | 15 |
| Thickness (mm) | 02 | 03 | 04 |
The Mg incorporation was done using 2 nd ingot. The furnace used for this experiment was a “well type” resistive furnace. The mold was heated in a muffle resistive oven at a constant temperature of 350 °C. The starting point for the casting of each batch of samples of the experiment was the 800 g of Al 50:50 ingot according to Table 2.
| Batch | Preparation |
|---|---|
| 1 st | 800 g. aluminum - (ingot 50 : 50) There were 3 runs*, obtaining 9 CP** |
| 2 nd | 800 g. aluminum (ingot 50 : 50) + 30 g. copper There were 3 runs, obtaining 9 CP |
| 3rd | 800 g. aluminum (ingot 50 % : 50 %) + 60 g. copper There were 3 runs, obtaining 9 CP |
| 4th | 800 g. aluminum (ingot 50 % : 50 %) + 60 g. copper + 40 g. of magnesium There were 3 runs, obtaining 9 CP |
| 5 th | 800 g. aluminum (ingot 50 % : 50 %) + 60 g. copper + 80 g. de magnesium There were 3 runs, obtaining 9 CP |
* Running means pouring the melt into the mold and
** CP stands for specimen.
After each batch of samples obtained in the experimental sequence one sample was taken. The analysis was performed using the optical emission spectroscopy technique, performed by a CCD Plus - S5 Solar Optical Spectrometer.
In order to reduce the thickness of the specimens from 2 and 3 mm to 0.5 mm, it was performed the rolling process, in the order of 4 x and 6 x, respectively.
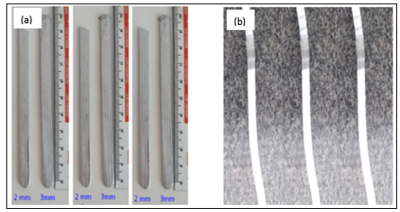
Figure 3: (a) Specimens after reduction and removing the mold.
(b) Laminated tape after reduction in the lamination process.
After that, the rolling process of the specimens was in the bench laminator to laminated tapes formation and in sequence the thickness of the laminated tapes (Figure 3) was validated. The reduction applied to the specimen in each pass was 38%, where the initial thickness was 4 mm. The specimens, after each pass in the laminator, were subjected to the annealing process to recrystallize and recover the mechanical properties, relieving the stress and tensions suffered in the lamination process.
A bench laminator with 10 x reduction capacity was used with a capacity receiving materials with a thickness of up to 5 mm and a minimum outlet thickness of 0.5 mm. The laminator has four pass routes, with approximately 40% reduction in each pass. Based on the initial measurement of the samples (4 mm) and the 40 % reduction capacity in each lamination pass and the final thickness (target 0.5 mm), four passes were made. The thickness measurements after each pass were recorded and statistically evaluated.
The final composition of the specimens after the addition of copper and magnesium can be seen in Table 3. We highlight that, at each run of molten material, 3 specimens were separated and the process was repeated 3 times in order to obtain the same specimen in triplicate, since each mold channel had a different thickness.
| Mixture Composition | ||
|---|---|---|
| Material | Massa (g) | (%) |
| Aluminum Cans | 400 | 42.55 % |
| Commercial Aluminum | 400 | 42.55 % |
| Copper powder | 60 | 6.38 % |
| Magnesium (99.9 % purity) | 80 | 8.51 % |
| Total | 940 | |
The electrical conductivity was measured in a digital contact conductivimeter, model Tecnatron DC-9. The conductivity calibration was performed with an aluminum standard (in 58.2 IACS at 20 °C). The measurements given are percentages of the conductivity of annealed copper according to the International Annealed Copper Standard (IACS). Thus, a material with 58.2 IACS corresponds to 58.2% of the conductivity of standard copper. For each formed alloy, three specimens were used and, in each sample, five measurements were made. For the conductivity result, the average of the three samples with five measurements was considered and for the degree of dispersion of the data in relation to the average, the standard deviation was applied.
The initial mass of 20 kg, acquired at the Santa Cruz do Sul Recycled Materials Collectors Cooperative - RS - Brazil, after slag removal, resulted in a 10.2 kg cast aluminum ingot. In percentage values, the yield obtained in the process was 51 % over the initial mass. It is noteworthy that the casting process was carried out in an industrial furnace with a combustion process that uses Liquefied Petroleum Gas (LPG) [16]. The research by Verran et al. obtained an average yield of 71.61 % through the use of an induction furnace. In addition, a scorifying flow was used in the process, which improved the yield to values above 80 % when 20 % by weight of scorifying flow was added [17].
For the chemical analysis of the cast aluminum ingot sample composition, which was composed of 50 % recycled aluminum and 50 % commercial aluminum, the optical emission spectroscopy technique was used. With the addition of alloying elements, copper and magnesium, to the 50:50 ingot, the casting was mixed and nine specimens were poured into the mold and a sample was taken for chemical analysis. The results of the analysis are shown in Table 4.
| Sample | Al (%) | Mg (%) | Mn (%) | Fe (%) | Cu (%) | Si (%) | Zn (%) | Cr (%) | Ti (%) | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Al cans + Comercial Al (50:50) | 98.2 | 0.787 | 0.305 | 0.283 | 0.101 | 0.242 | 0.028 | 0.020 | 0.000 | |
| Al alloy (50:50) plus 60 g Cu | 92.21 | 0.69 | 0.323 | 0.343 | 4.176 | 0.593 | 1.569 | 0.014 | 0.026 | |
| Al alloy (50:50) plus 60 g Cu and 80 g Mg | 82.79 | 6.775 | 0.368 | 0.379 | 7.00 | 0.564 | 1.96 | 0.012 | 0.025 | |
| Al cans [30] | 97.90 | 0.626 | 0.732 | 0.358 | 0.113 | 0.145 | 0.03 | 0.02 | 0.02 | |
| Data content - alloys used in the manufacture of Al cans [17]. | ||||||||||
| Body | - | 0.8-1.3 | 1.0-1.5 | 0.70 | 0.25 | 0.30 | 0.25 | - | - | 0.05 |
| Lid | - | 4.0-5.0 | 0.2-0.5 | 0.35 | 0.15 | 0.20 | 0.25 | 0.10 | 0.10 | 0.05 |
| Seal | - | 3.3-4.0 | 0.25-0.4 | 0.35 | 0.15 | 0.20 | 0.25 | 0.15 | 0.10 | 0.05 |
Conditions: 800 g of alloy, casting by gravity and cast child 450 °C
The results show that the aluminum contained in the manufactured alloys with recycled cans was very close chemical composition of reference Al alloy. The percentages of manganese are below the reference. As for the other elements that appear in the form of impurities, it can be stated that the values found are within the limits allowed in all standards that define chemical composition specifications for workable aluminum alloys. It should be noted that data from Verran et al. cited in the table were obtained only with recycled aluminum cans [18].
According to Jerina et al. the quality of recycled material depends on several factors, including material purity, coating types and size [19]. The control of impurities has a major influence on the mechanical properties of recycled alloys. Similarly, Mansurov et al. mention that recycling causes individual and combined addition of impurity elements, such as Si, Fe, Cu, Zn, Pb, Sn, Ni and Mn, in the alloy casting properties [14, 20].
Iron is the most commonly found impurity element in aluminum recycling because it is very difficult to remove and gradually builds up through repeated recycling [21]. Together with aluminum and other alloying elements such as manganese, copper, magnesium and silicon, iron produces intermediate phases, which are detrimental to the mechanical properties of the final product during solidification [22]. Ashtari et al. reinforce that iron is one of the most problematic impurities in aluminum castings [23]. Zhang et al. present that the addition of a suitable neutralizer such as Mn, Cr, Be, Co, Mo, Ni, V, W, Cu, Sr or other rare earth elements such as Y, Nd, La and Ce could control the deleterious effect of Fe in aluminum alloys [24].
The process of re-melting discarded cans has a prominence in the aluminum cycle production chain in Brazil [16]. The can is composed of body, seal and lid, each of which is made up of different alloy compositions. The can body is made from ASTM 3004 aluminum alloy, the lid is made from ASTM 5182 alloy and the seal is made from ASTM 5082 aluminum.
According to magnesium, when subjected to high temperatures, undergoes variations in casting processes when in contact with oxygen, unless care is taken to protect the surface against oxidation [5]. This protection occurs by the use of inert gas injection such as argon on the casting surface. Similarly, iron has a 72.5 % variation between highs and lows. Contaminant elements such as Ti, Pb and Cr can be found in small quantities in the recycled aluminum [20].
The addition of alloying elements adds characteristics to aluminum to provide the melt with properties of interest. Since the objective of this research is to produce a conductive laminated tape, it is relevant to add alloying elements such as copper, as its conductive properties are superior to aluminum. In addition, copper has better tensile strength and corrosion resistance, increased hardness, higher ductility and conformability [25]. Magnesium is another element that has properties that may favor the alloy composition to form the conductive tape. To providing mechanical gains, magnesium allows the alloy to maintain a high level of corrosion resistance and weldability. However, magnesium is highly soluble at its melting point and it must be melted in an argon controlled atmosphere [26]. Al-Mg alloys with contents ranging from 3 % to 5 % form alloys such as 5042, 5352, 5082 and 5182, which are used in the manufacture of beverage can lids [20].
Therefore, molten aluminum from Al recycled cans was applied to manufacture laminated tape. Alloy elements such as copper and magnesium were added to this aluminum, which resulted from the second casting. The research evaluates whether these elements add improvements in the conduction properties of electric current in laminated tape.
In the composition, considering the all experimental essays, there is a proportional increase in the addition of copper to the melt (50:50 ingot), which was successful, representing 4.176 % of the alloy. The next addition was made with the incorporation of 40 g of magnesium. There was a slight contamination of impurities from the crucible wall, which resulted in an increase in zinc, with a value of 1.569 % in the alloy
Next, a new casting was made, an additional 30 g of copper and 80 g of magnesium were added. Due to the addition of magnesium, argon gas injection was used to control oxygen contact in the bath since magnesium is highly soluble when in contact with oxygen. After stabilization of the bath and homogenization of the alloy, the argon was turned off and the melt was poured back into the mold and heated at 350 ° C. With the addition of copper and magnesium, an improvement in fluidity was noted and, as a result, the channels were completely filled. This left the specimens at the size determined by measuring of the mold channel.
The percentages of copper and magnesium were 7 % and 6.775 % respectively. Magnesium did not reach the predicted percentage of 8 % from Table 1 due to the degree of solubility and an increase in slag formation on the surface of the melt caused by magnesiumoxidation. Copper behavior remained stable as in previous flows. The other elements remained practically the same in relation to the previous additions made. Based on that, Al cans recycling are good choices for the development of high value products. Of course, when it comes to recycling, impurities will always exist in the casting process, which will contribute to slag formation. Thus, researching casting methods or techniques that improve process performance is the first challenge for researchers interested in the topic of metal recycling.
The measurement of the electrical conductivity of the samples was performed after each lamination pass presented in Table 5 was performed.
| Lamination order | Thickness (mm) | Redution (%) | Al (50:50) | Al (50:50) + Cu | Al (50:50) + Cu + Mg |
|---|---|---|---|---|---|
| Molten | 4.04 | 0.00 | 47.67 | 46.98 | 46.68 |
| 1st pass | 2.58 | 36.14 | 47.29 | 46.67 | 46.37 |
| 2nd pass | 1.60 | 60.40 | 47.06 | 46.49 | 46.19 |
| 3rd pass | 0.99 | 75.50 | 46.91 | 46.38 | 46.07 |
| 4th pass | 0.59 | 85.40 | 46.87 | 46.34 | 46.03 |
A drop in the electrical conductivity of the alloys is observed as the cold rolling process increases. In the same way, it occurs that with the addition of Cu and Mg over the aluminum sample (50:50) as a base [27]. A similar study was carried out by Kotiadis et al. using aluminum alloys with transition metals such as Fe and Ni that have the potential to form alloys with high electrical and thermal conductivity for heat dissipation applications. The cast alloy showed 48.83 ±0.31 pct IACS.
In the research by Koprowski et al. a percentage of 61.1% of IACS conductivity was obtained using an aluminum alloy with the addition of 0.2% by weight of Mg, Co and Ce to technically pure aluminum [28]. The study showed that an increase in deformation leads to an increase in the resistance properties of the material and a decrease in the electrical conductivity, but different additives have different effects on these properties.
The process of casting recycled aluminum shows a wide possibility for reusing solid waste. In addition to providing income to support many families of waste pickers in urban centers, recycled aluminum can be used for various industrial applications. The sustainability bias brings another important point when considering the recycling of materials, since the continued depletion of minerals from the earth’s crust is a well-known fact. For this reason, this research is relevant. Recycling aluminum allows the reuse of materials and reduces the need for underground mining. In addition, this work reached a more appropriate destination for industrial waste, therefore offering possibilities for saving materials as in our conductive Al tapes production.
Casting recycled aluminum offers possibilities for a new application, simply by adapting matrices or molds to the development. There is a possibility to expand the methodologies and obtain new applications of aluminum casting using clean technologies that promote greater sustainability can be advanced.
The addition of copper and magnesium to the molten aluminum for the ingot 50:50 has shown a great possibility for the casting of different materials with developed methodologies. This was demonstrated in this research through the production of a new alloy where 85 % aluminum, 6.38 % copper and 8.51 % magnesium were obtained with element composition recognized as suitable for electrical conductivity and with the lamination until 0.5 mm was possible.
The electrical conductivity results of this research recorded values of 47 IACS. It is recommended that, for future research, only recycled aluminum be used and, on top of this, optimize the casting processes, through the use of a scorifying flow, which will provide a better mass yield of the aluminum obtained by smelting by beverage cans.
For other applications or even to further improve the ability to conduct electricity, it is recommended to carry out studies with recycled aluminum using as reference 6xxx series alloys, such as alloy 6101, used in the manufacture of electrical conductors and overhead cables in electrical networks performed according to Karabay and others authors [29-35].
To National Research Council (CNPq) process 310228 / 2019-0 and 303934 / 2019-0.
We hereby declare that this submission is our own work and to the best of our knowledge it contains no materials previously published or written by another person.
Gilson Gilmar Holzschuh: Conceptualization, Methodology, Validation, Writing - original draft, Visualization, Formal analysis, Investigation.
Jorge André Ribas Moraes: Supervision, Validation, Writing - review & editing.
Sergio Boscato Garcia: Validation, Writing - review & editing.
Liane Malmann Kipper: Validation, Writing - review & editing.
Rosana de Cássia Schneider: Validation, Writing - review & editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
*This study was funded in part by the National Research Council (CNPq) process no 310228 / 2019-0 regarding the productivity grant granted to researcher Rosana C. S. Schneider, and by processno 303934 / 2019-0 regarding the productivity grant granted by the National Research Council (CNPq) to researcher Liane M. Kipper.
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study
