Author(s): Malek Michael Bouhairie, Sabrina Nasreddine, Racha Seblani and Hassan Akouch*
Valproate induced hepatotoxicity is a well-known side effect, which frequently required periodic monitoring of serum drug level. Hepatotoxicity caused by valproate typically occurs at supratherapeutic drug levels. Once in a while, an idiosyncratic reaction is elicited, liver injury might occur despite normal serum valproate level mainly in chronic users. We hereby identify an unusual case of acute idiosyncratic valproate induced hepatotoxicity. We report a case of a 65 years old male with dyslipidemia and history of seizure, on valproic acid therapy, presented with altered mental status and drowsiness. The patient’s home medications include only zenil 10 mg daily and valproate which was started one month ago. At presentation, He was awake, oriented, but lethargic. Laboratory testing reveals hepatocellular injury with elevated transaminase levels, direct hyperbilirubinemia and coagulopathy. The ammonia level was normal and valproate level was within the therapeutic range. Abdomen computed tomography with IV contrast and MRCP results were irrelevant. Idiosyncratic valproate toxicity was diagnosed after exclusion of all other possible etiologies and after a rapid clinical and laboratory improvement once the drug was discontinued. Based on the patient’s clinical context the diagnosis of valproate induced hepatotoxicity was confirmed. This case emphasizes the importance of identifying, diagnosing, and managing valproate toxicity when no alternative clarification for their symptoms. We need further attempts and more researches to improve the detection of adverse hepatic reactions and to obtain reliable information about the discovery of new biomarkers or tools for early prediction of DILI, as well as to obtain accurate information on epidemiology, drug safety, and pathogenesis in order to improve management for better survival.
Valproic acid (VPA) has been by far one of the most common & widely used broad-spectrum anticonvulsant medication worldwide as an effective treatment for generalized seizures, focal seizures, major depression, bipolar disorders and migraine prophylaxis [1].
Several prospective studies discussed plenty of Valproate’s side effects like insulin resistance, obesity, neurological, hematological & metabolic disorders,they also showed that valproic acid is responsible for hepatotoxicity. The majority of these direct hepatotoxicity occurs when the drug’s supratherapeutic level is reached in the blood. Raised transaminase levels have been accounted for in up to 5-10% of patients, while hyperammonemia is more prevalent with 27.8% of patients [2,3].
Hyperammonemic encephalopathy is a popular reaction of valproic acid inconsequential to hepatotoxicity with normal transaminase levels [4].
Other uncommon reactions have been accounted for, one of which is discussed in our patient; idiosyncratic liver injury induced by valproic acid within one month of exposure. This is in reality an uncommon occasion, in spite of the fact that it can often be fatal. Frederick J. Suchy et al.report two fatal cases of acute hepatic failure associated with the use of sodium valproate [5]. Zaccara G et al. also reported a case of reversible fulminant Depakeneinduced hepatotoxicity that occurred at the therapeutic drug level and was ascribed to idiosyncrasy [6].
Idiosyncratic reaction is defined as side effects that cannot be explained by the offending agent’s established mechanisms of action, occurring at variable doses in most patients and vaguely only in vulnerable individuals. Up to 10% of all adverse drug reactions are commonly thought to account for these reactions, but they can be more frequent, depending on the accepted definition. VAP induces hepatic toxicity at both dose related and nondose related; dose related toxicity is common and observed in approximately 11% of supervised patients, it usually presents as mild increase of liver transaminases with mild or no systemic symptoms, it completely resolves with drug withdrawal or even dose reduction .While, idiosyncratic or non-dose related liver injury is rare ,around one of every 20000 [7,8].
Drug-induced hepatotoxicity or DILI leading to acute liver failure in patients has become the main reason for liver transplantation in the United States [9,10]. It additionally has been the most usual single explanation behind pulling out approved medications from the market [11,12].
We hereby report a case of reversible fulminant valproate-induced hepatotoxicity in 60-year-old male patient with a medical history of seizure, who presented with elevated liver transaminases secondary to VPA intake as a consequence of an acute adverse drug reaction and was attributed to idiosyncratic liver injury known as DILI.
A 65-year-old male, non-smoker, healthy (he walks 1 hour daily), was brought to the emergency department for gait imbalance. The patient was well until one week after starting depakine for his seizure when notable drowsiness and malaise developed. He complained of increased fatigue, sleepiness and anorexia progressively. The next following days, he had episodes of multiple alternating non-bloody diarrhea and constipation, accompanied by abdominal distention, diffuse vague abdominal pain, and xerostomia. The patient continued his medication without seeking medical advice; he claimed that he had dark tea-coloured urine.
PMH: he had dyslipidemia, and long term before admission he had taken zenil (lipids lowering agent) 10 mg once daily. He had developed syncope four years ago, lasting few seconds without postictal phase, as the patient did not seek medical advice; another two similar witnessed episodes reoccurred in March and September 2020, both lasting 10 minutes, the last episode required hospitalization in October and full workup was done: echocardiography, HBA1c, TSH and labs were normal; MRI brain reveals very subtle loss of digitization on the left hippocampal head without any loss of volume or alteration in the signal intensities, suggestive of the possibility of very early hippocampal sclerosis and recommend EEG, which the later show abnormal epileptogenic waves in the right frontotemporal lobe, require the initiation of valproic acid on December 05, 2020. PSH: Previous cholecystectomy, colonoscopy in 2015 where is nothing pertinent.
He denies fever, no travel history, no alcohol or herbal supplements intake, no known contact with sick persons, he had no drug or food allergies, no risk factors for viral hepatitis, and no family or personal history of liver disease.
Few days before admission, his family noted yellowing of the eyes and the skin, they considered to stop the valproic acid immediately. Valproate was stopped, and he was admitted to the Sahel hospital 3 days later because of worsening jaundice, exacerbation of his fatigue and xerostomia.
Upon presentation to the emergency department , the vital signs comprised a temperature of 36.1 °C, blood pressure was 117/73 mmHg, the heart rate =70 beats per minute, the respiratory rate was 15 breaths per minute and oxygen saturation was 94% while the patient was breathing ambient air. PCR Covid 19 was negative, chest x-ray show non infiltrate Figure 1, ECG: sinus rhythm.
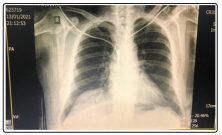
Figure 1
On examination, he was alert; conscient, cooperative, oriented to place, person and time; looking well with mild distress; the skin was jaundiced and the sclerae were icteric. The abdomen soft, positive bowel sounds, mild vague diffuse tenderness mainly on RUQ, no organomegaly, no signs of chronic liver disease(no palmar erythema ,no JVD...),no skin lesions, no asterixis; nothing pertinent on the rest of the physical exam. Rectal examination revealed brown stool that tested positive for fecal occult blood.
He was placed in the inpatient medicine service where he was seen for evaluation, laboratory tests revealed hepatocellular injury, elevated liver enzymes: ALT=1816 U/L, AST= 1278 U/L, Alkaline phosphatase = 166 U/L, GGT= 114 U/L, prothrombin time 18.3 seconds, prolonged INR=1.77 and elevated total serum bilirubin = 9 mg/dl; ammonia level was normal and valproate level was within the normal range Table 1.
| January 2021 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 25 | 26 | 27 | 28 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine mg/dl | 0.6 | 0.6 | 0.6 | 0.7 | 0.8 | 0.6 | 0.7 | 0.8 | 0.8 | |||
| Total bilirubin mg/dl | 9.7 | 10.7 | 14.2 | 15.7 | 16.6 | 17.4 | 20 | 21 | 24 | 22.7 | 26 | 21.4 |
| Direct bilirubin mg/dl | 6.2 | 6.5 | 8.5 | 9.7 | 9.8 | 10.6 | 11.6 | 12.8 | 13.4 | 13.1 | 14.2 | 12.1 |
| SGOT IU/L | 1059 | 1019 | 1083 | 894 | 1041 | 1040 | 1083 | 1020 | 759 | 773 | 734 | 539 |
| SGPT IU/L | 1744 | 1604 | 1747 | 1520 | 1637 | 1588 | 1589 | 1515 | 1018 | 967 | 910 | 655 |
| GGT IU/L | 114 | |||||||||||
| Alkaline phosphatase IU/L | 183 | 138 | ||||||||||
| Albumin g/dl | 3.2 | |||||||||||
| INR | 1.77 | 2.58 | 3.33 | |||||||||
| WBC | 6.39 | 8.75 | 8.68 | 5.53 | ||||||||
| Platelet (*103) | 188 | 212 | 194 | 98 | ||||||||
| Hemoglobin g/dl | 13.3 | 14 | 13.2 | 14.1 | ||||||||
| LDH | 450 | 286 | 296 |
Tests for autoimmune (ANA, AMA, ASMA and Anti-LKM) and viral hepatitis (HAV, HBV, HCV, EBV and CMV) were unremarkable; also protein electrophoresis and TSH were normal.
Scanner abdominal with iv contrast demonstrated a homogenous liver with normal size and normal contour, no nodularity ,no intra or extra hepatic ducts dilation ,gallbladder not visualized (previous cholecystectomy ) no evidence of biliary obstruction, kidney and spleen were normal Figure 2.
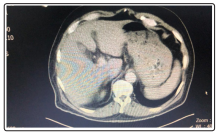
Figure 2
MRCP showed minimal heterogeneous fatty infiltration of liver, minimal intrahepatic biliary tree dilation is seen with heterogeneous likely beaded appearance, CBD not dilated reveals no intra-luminal stone Figure 3.
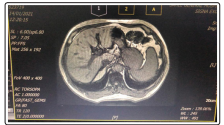
Figure 3
Given the patient context, we suspected a drug induced liver failure secondary to valproate. Hence, we keep depakene stopped and IV hydration with Normal saline 3 litres per 24 hours started, with 400mg q6h of N-acetylcystein orally and 1 vial of albumin 20% IVD per 12 hours; In addition, lactulose, ursofalk started with PPI prophylaxis.
The patient recovered slowly, his liver function tests have experienced an upward trend, however his clinical symptoms had improved, expressed by significant amelioration of his gastrointestinal upset.
By the seventh day, his total serum bilirubin raised steadily to reach a peak of 21 mg/dl, then experienced a gradual increase to hit a maximum point of 26 mg/dl in 14 days.
While his ALT and AST declined slowly from 1816 IU/L and 1278 IU/L to 1515 IU/L and 1020 IU/L respectively by 7 days to reach 655 IU/L and 539 IU/L in 2 weeks later.
Based on laboratory trending and clinical course, the final diagnosis of valproate induced liver injury was made .In our patient, regarding the temporal relation of valproate intake and the clinical symptoms, adverse drug reaction was strongly suggestive and therefore no further investigation for other etiologies was required .
During his hospital stay ,the patient started to have fever 10 days later ,his oxygen requirement increased, chest x-ray revealed new bilateral ground glass opacity. Urgent PCR Covid-19 was done Figure 4, he was diagnosed with covid19 pneumonia which rapidly deteriorated and was complicated by septic shock leading to death.
Figure 4
Acute liver failure is an uncommon but life-threatening critical illness that happens frequently in patients who don’t have previous liver disease, most commonly seen in healthy adults in their third to fourth decade [13]. Fortunately, its incidence is assessed to be 14 to 19 cases for each 100,000 people, with jaundice going with 30% of patients [14].
According to the American Association for the Study of Liver Diseases (AASLD), acute liver failure is defined as severe hepatic dysfunction that is characterized by rapid onset and incorporate elevated transaminases, coagulopathy (international normalized ratio more than 1.5) with or without encephalopathy in a patient without preexisting liver disease and with an illness duration of less than 26 weeks. That may be complicated with multiorgan failure leading to death in up to a large percentage of the patients [15-16].
Acute liver failure may be fulminant or subfulminant, fulminant liver failure is characterized as hepatic injury without pre-existence disease, possibly reversible followed by hepatic encephalopathy within 2 months after the initial symptoms [17]. This definition remains applicable nowadays, but it is slightly upgraded to take in consideration the period between the onset of symptoms and the development of encephalopathy (latency). This interval gives hints to the reason for illness, likely complications and prospective prognosis with only supportive care [18]. It is classified as hyperacute cases, when this period is around a week or less and often caused by viral hepatitis or acetaminophen toxicity. More gradually illness or subacute cases, sometimes confused with chronic liver disease usually precipitated by idiosyncratic drug induced hepatic injury or may be of unknown etiology, patient with subacute disease surprisingly had worse outcomes if left untreated notwithstanding having less significant coagulopathy and encephalopathy compared with those had more rapid onset.
Drug-induced liver injury (DILI) has become a major public health concern in recent years, owing to widespread human exposure to pharmaceuticals and herbal medicines. The cost of DILI is expected to rise. Given the increasing commercialization of new pharmacological molecules, the use of multiple medications in aging population, and the growing consumer trend of herbs and dietary supplements ,the burden on DILI is expected to increase further in the future. In addition, DILI is the first cause of acute liver failure in the United States and Europe and is ranked as the leading cause of premarket and postmarket regulatory measures.
Notably, during the years 1969 and 2002 fifteen percent of drugs withdrew from the market due to an outbreak of liver toxicity, and also eight drugs were withdrawn in Europe and in the united state between 1997 to 2016 for the same reason to classify these risk in drug production, predictive algorithms and targeted preclinical testing are recommended to identify to what extent the drug is reliable. In addition, as previously mentioned, new biomarkers and pharmacologic models are being researched in order to predict and enhance DILI detection and risk assessment [19].
Drug-induced liver injury (DILI) is the primary cause of acute liver failure; 5,484,224 participants were included in a retrospective population-based cohort study performed in Northern California, showing that 9.3 percent of patients were given a diagnosis of definite or probable ALF, where 52 percent of them had predominantly acetaminophen or DILI etiology [20]. Druginduced liver injury (DILI) usually occurs within six months of drug initiation. Medications regularly involved in instances of DILI incorporate antimicrobials, anticonvulsants, and non-steroidal antiinflammatory drugs, not to mention dietary supplements and herbal medications. Most cases of hepatotoxicity are idiosyncratic and therefore unpredictable and making them difficult to diagnose and prevent, Although there appears to be a dose threshold for each specific agent. It is one of the most difficult diagnoses faced by clinicians due to the incredible number of drugs that are able to damage the liver, as well as the variety of their phenotypes and basically the current lack of diagnostic biomarkers [19].
In the United States drug-induced hepatic injury accounts roughly for up to 50 % as the main reason for liver dysfunction [21]. Such damage could be direct, predictable, reproducible in animal models with short latency periods of one to five days and contingent on the dosage as illustrated by Acetaminophen-induced direct hepatotoxicity, which is the most frequent reason for acute liver failure. It’s going to even be idiosyncratic, unpredictable and potentially dose-independent like our patient, not reproducible in animal models with variable latency period. However indirect injury is arising as a third type , result from the mechanism of action of the drug to induce or exacerbate pre-existing liver disease ,it may be secondary to entire class of drugs (tumor necrosis factor antagonists and checkpoint inhibitors) and with different phenotype for instance: fatty liver disease caused by medications that alter triglyceride disposition, insuline sensitivity, or cause weight gain; acute hepatitis via Anticancer chemotherapeutic agents that trigger reactivation of hepatitis B or antiretroviral agents that cause immune reconstitution and exacerbation of hepatitis C . It is preventable and is much more common than idiosyncratic reactions to a specific agent [22].
Idiosyncratic liver damage caused by medications is uncommon, despite concomitant exposure to hepatotoxic drugs with small percentage progress to ALF or encephalopathy typically after one of every 2000 to 1/100000 cases exposures [23-24]. It is classified as hepatocellular, cholestatic, or both (mixed) according to the R ratio; which is determined by dividing the alanine aminotransferase level by the alkaline phosphatase level from the time of initial presentation, with both values are expressed as multiples of the upper limit of the normal range, an R value of more than 5 indicates hepatocellular damage, a value of less than 2 indicates cholestatic injury, and a value of 2 to 5 indicates mixed injury. The calculated R ratio in our case assumed to be = (1744/42) ÷ (183/135) = 30 representing an hepatocellular injury
Acute hepatocellular hepatitis is a common manifestation of idiosyncratic liver injury. The latency period usually lasts 5 to 90 days. The clinical course and symptoms are similar to those of acute viral hepatitis, in which alanine amino-transferase significantly increased from 5 to 50 times the upper limit of normal, while alkaline phosphatase slightly increased to note that mortality rate from icteric hepatocellular injury due to drugs is high, usually 10% or more, a feature first highlighted by the late Hyman J. Zimmerman, hence the name Hy’s rule. Jaundice caused by hepatocellular injury rather than cholestatic damage is a key feature of Hy’s rule. Our patient met this criteria [19].
The etiology of idiosyncratic DILI is multifactorial, and a number of internal and external factors influence the development of adverse events in vulnerable individuals, There are various risk factors associated with DILI, such as host factors (age, sex, genetics) ,drug-dependent factors(some drugs properties increase the risk of liver injury like daily dose of more than 50 mg, more than 50% of liver metabolism, the higher the lipophilicity and/or dual inhibition of mitochondrial and bile salt export pump...) and environmental factors . Multiple factors are related to increased risk of mortality such as older age, elevateted transaminase levels, bilirubin and coagulopathy [25].
The diagnosis is especially challenging, since no specific diagnostic markers and it is primarily based to a great extent on exclusion of other etiologies using special investigations such as testing for serology markers, imaging or liver biopsy and establishing a compatible temporal relationship between medication consumption and the emergence of clinical or laboratory indications/markers of liver damage .
Therefore the main diagnostic components are : latency, onset of symptoms concomitant with drug exposure; dechallenge, withdrawal of symptoms with abstinence of drug; rechallenge ,recurrence with reexposure; likelihood, probability of the agent ‘s potential for liver injury; and phenotype ,the clinical context [26-27].
VPA is a simple structure of eight-carbon branched chain carboxylic acid with weak acidic properties; which has been credited to blockage of voltage-gated sodium channels and increased brain levels of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter [28]. It is primarily used to treat generalized seizures, focal seizures, major depression, bipolar disorders and also used for migraine prophylaxis. Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the first 6 months of treatment. Serious or fatal hepatotoxicity may be preceded by nonspecific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first 6 months.
When considering encephalopathy in the setting of VPA, DILI is often overlooked, physicians ought to consider DILI in the setting of hyperammonemia and transaminitis.despite hepatotoxicity is a well-reported side effect of valproate, VPA is generally well tolerated with little side effect, especially when the initial dose is slowly titrated. Nonetheless the toxic levels of VPA can cause depression of the respiratory and the central nervous system. There have been reports of gastrointestinal effects, including nausea, vomiting, diarrhea, and pancreatitis which is worst(most prominent) in children over 2 years of age, patients receiving numerous anticonvulsants, as well as patients with concomitant liver disease [29].
Concerning valproate metabolism is extensively hepatic via glucuronide conjugation (30% to 50% of administered dose) and 40% via mitochondrial beta-oxidation; other oxidative metabolic pathways occur to a lesser extent. Clearance is decreased with liver impairment. Hepatic disease is also associated with decreased albumin concentrations and 2- to 2.6-fold increase in the unbound fraction. Free concentrations of valproate may be elevated while total concentrations appear normal. Periodic monitoring of liver enzymes (at baseline and frequently during therapy especially during the first 6 months), CBC with platelets (baseline and periodic intervals), PT/PTT (especially prior to surgery), serum ammonia (with symptoms of lethargy, mental status change), serum valproate levels; suicidality (eg: suicidal thoughts, depression, behavioral changes); motor and cognitive function (for signs or symptoms of brain atrophy).
Some experts have speculated that a drug-induced carnitine deficiency that can precipitate mitochondrial dysfunction is involved in the underlying mechanism. Consequently, the administration of the medication l-carnitine can be effective both for those who are chronically taking valproate and for those who experience the effects of an acute overdose of valproate. Bohan TP et al. analysed the relationship between treatment with L-carnitine and liver survival in 92 patients ,with severely symptomatic valproate-induced hepatotoxicity.They found that the greatest liver survival was significantly associated with early IV administration of L-caritine treatment especially within 5 days of diagnosis rather than enteral L- carnitine or supportive care only with p value less than 0.001. The following is the rationale for using l-carnitine in valproate-induced hepatic dysfunction: Carnitine helps to maintain the ratio of acyl to free coenzyme A by facilitating fatty acyl group transfer into the mitochondria. if an overdose of valproate is taken , Valproate is not completely metabolized and the compound valproyl CoA accumulates. The treatment with L-carnitine tends to reverse and even protect against this process [30]. Since most drug-induced hepatotoxicity is handled with the removal of the inciting agent, this method is special. Since the mortality rate in these cases is always very high, using l-carnitine appears to be both scientifically rational and clinically beneficial.
Although The impact of valproic acid on fatty acid metabolism by inhibiting fatty acid β-oxidation seems to be a factor in the mechanism of hepatotoxicity. furthermore , some hypothesis about excessive oxidative stress and genetic variants of some enzymes, such as CPS1, POLG, GSTs, SOD2, UGTs and CYPs genes, have been reported to be involved with VPA- hepatotoxicity.bedside, Carnitine supplementation and administration of antioxidant agents were also proved to be effective strategies for treating liver damage induced by valproate [31].
Patient treated with valproic acid should be assessed for hyperammonemia and should check regularly the LFT’s and valproate level, because despite idiosyncratic reaction is rare which can occur with normal valproate level can be fatal, that’s why should be assessed early and keep in consideration for early intervention , when early recognition and incite withdrawal of sodium valproate may prompt clinical improvement in DILI. Patient treated with valproate, showing features of intellectual disability, focal neurological deficit, sleepiness or drowsiness, should be surveyed for hyperammonemia and liver transaminase tests.
Patients with hyperacute liver failure(acetaminophen/ischemic hepatopathy ) tend to have a better prognosis than those with subacute liver failure, no specific treatment after discontinuation of the offending agent were recommended .Awareness about VPA toxicities can guide patient therapy toward the right direction in critical patient situations and will help improve patient survival. There is a very limited evidence base for supportive therapy due to the rarity of acute liver failure and its severity and heterogeneity, but survival rates have improved significantly in recent years along with advances in critical care management and the utilize of emergency liver transplantation [32,33].
Every patient on valproic acid should be assessed for hyperammonemia and should check regularly the LFT’s and valproate level; because despite idiosyncratic reaction is rare which can occur with normal valproate level, it can be fatal, that’s why the patient should be assessed early and keep in consideration for an early intervention with emergent multidisciplinary approach to assess for liver transplantation which is the only curative treatment. Regular monitoring of therapeutic drugs during VPA treatment and periodic strict monitoring of hepatic biochemistry, as well as VPA. Genotype screening for specific patients at high risk prior to administration may improve the safety profile of this anti-epileptic drug. This case emphasizes the importance of identifying, diagnosing, and managing valproate toxicity when no alternative clarification for their symptoms. We need further attempts and more researches to improve the detection of adverse hepatic reactions and to obtain reliable information about the discovery of new biomarkers or tools for early prediction of DILI, as well as to obtain accurate information on epidemiology, drug safety, and pathogenesis in order to improve management for better survival.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to thank the doctors and staff at our institution for their continuous support, insights and guidance.
