Captopril and Hydrochlorothiazide: Insights on Pharmacology and Analytical Chemistry Profile
Author(s): Mahmoud M. Sebaiy*, Karim M. Hegazy, Alzahraa M.F. Ebrahim, Fatma M. Essam, Fatma A. Amin, Fatma H. Bakry, Farouk R. Farouk, Fatma H. Eldossoki, Fatma E. Amer, Fatima S. Abdelazim and Samar S. Elbaramawi
Abstract
Many categories of drugs are used today for hypertension such as captopril which belongs to ACE inhibitors family and hydrochlorothiazide which consider a diuretic drug.In this literature review, we will focus on their pharmacological effect as well as most of the recent reported analytical methods that have been established for their determination in their pure form, combination form with other medications, combined form with its metabolites, and in biological materials.
Introduction
One of the most common diseases in the world is Hypertension,
which is usually defined as persistent blood pressure (BP) of
140/90 mm Hg in the medical office, and it is one of the leading
causes of premature morbidity and mortality in the United States
[1,2]. Hypertension has no recognized cause and raises the risk
of brain, cardiac, and renal problems. In developed countries, the
chance of getting hypertensive (blood pressure >140/90 mm Hg)
during one's lifespan is greater than 90%. Other cardiovascular
risk factors such as age, obesity, insulin resistance, diabetes, and
hyperlipidaemia frequently coexist with essential hypertension [3].
Many categories of drugs are being used to control hypertension
as a diuretic, calcium channel blocker (CCB), angiotensinconverting enzyme (ACE) inhibitor, beta-blocker, and angiotensin
receptor blocker (ARB). Moreover, even though diuretics were
first used to treat hypertension nearly five decades ago, they
are still an important therapy option today. Despite the fact that
their popularity as preferred antihypertensive medications have waned, diuretics are still routinely used to treat hypertension,
either alone or in combination with other types of drugs [4].
Thiazide diuretics as hydrochlorothiazide are the most commonly
prescribed diuretics for hypertension, but other classes of diuretics
may be useful in alternative circumstances. Although diuretics
are no longer considered the preferred agent for the treatment of
hypertension in adults and children, they remain acceptable firstline options [5]. In addition, ACE inhibitors, like Captopril, a drug
that has been widely used to treat hypertension and congestive
heart failure in individuals, which inhibit ACE activity and thereby
reduce the synthesis of angiotensin II, are also used. Furthermore,
ACE inhibitors limit the breakdown of bradykinin, enhancing its
vasodilatory and other effects [6,7].
Pharmacology
Captopril (CAP) (figure 1), is an (ACE) inhibitor, and it has
been shown in animal and human trials to reduce left ventricular
remodeling (structural enlargement and alterations) following
myocardial infarction, which can lead to left ventricular
dysfunction and an increased risk of death. In several animal
studies, ventricular remodeling(structural changes such as infarct
expansion and thinning caused by stretching of the infarct zone
and rearrangement of myocytes) was reduced after myocardial
infarction, and survival in the rat model of myocardial infarction
was significantly improved.
These findings have now been verified in human trials and are
thought to be the result of CAP's balanced reduction in preload
and afterload, other processes such as a reduction in coronary
blood flow, increase prostaglandin synthesis, limit catecholamine
release, and potentiation of bradykinin action [6, 8, 9]. CAP lowers
plasma angiotensin II and raises angiotensin I concentrations, and
this leads to increasedplasma renin activity or renin concentration,
and decreased aldosterone concentration or urinary aldosterone
excretion, respectively [10]. CAP has an oral bioavailability of
around 60% in healthy fasting volunteers, and co-administration
of food or antacids lowers CAP bioavailability by 25 to 50%.
The peak plasma CAP concentration at 1 hour after delivery [6].
Hydrochlorothiazide (HCT) (figure 1)is an anti-hypertensive
diuretic drug, it does its action via the prevention of sodium
reabsorption as it blocks the membrane [11, 12]. Stimulation of
the renin-angiotensin-aldosterone (RAAS) and sympathetic nerve
systems are results of the decrease in cardiac output caused by
thiazide-associated volume depletion, leading to progressive salt
and NCCT (the electroneutral sodium-chloride cotransporter)
which is founded on the distal convoluted tubule's apical water
retention. The compensatory salt and water reabsorption brings the ECF volume to baseline after 4-6 weeks. Surprisingly, thiazide's antihypertensive impact persists despite normalization of ECF
volume due to a decrease in peripheral vascular resistance [13, 14]. The factors involved for vasodilation and long-term blood pressure
reduction are unclear, but they appear to involve both a direct and indirect action on the vascular endothelium and/or muscular [15].
It has a lower duration of action and is less potent than ACE inhibitors [16, 17]. Moreover, hydrochlorothiazide Increases hydrogen
and potassium ion secretion and calcium reabsorption as it increases the expression of a sodium-calcium exchange channel [18].
Thiazide diuretics can be given once daily or every other day in some cases. The initial dose of hydrochlorothiazide can range from
6.25 to 12.5 mg per day, with some people later requiring doses of up to 25 to 50 mg per day. Thiazides lose efficacy when excessive
salt is consumed, in patients with renal failure, and in patients using nonsteroidal anti-inflammatory medications [19].
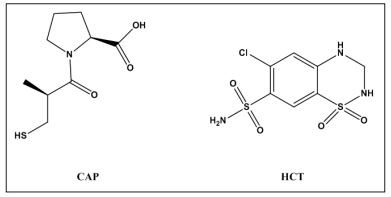
Figure 1: Chemical structures of captopril (CAP) and hydrochlorothiazide (HCT)
We have reported before review articles for many analytical techniques that have been used for the determination of important drugs
in different forms [20-49]. As such, to continue our strategy of reviewing the analytical methods, in this review article, CAP and
HCTwhich are usually prescribed as a combined dosage form have been studied in respect of pharmacology, mode of action and most
reported analytical methods that have been developed for determination of both drugs in different matrices.
Analytical Methods
1. Capillary electrophoresis methods:
| Drugs |
Matrix |
Capillary |
Buffer (base electrolyte) |
Detector |
Linearity range |
LOD |
Ref |
CAP, HCT and
their impurities |
Tablets |
Fused-silica capillary
(50 μm inner diameter,
375 μm outer
diameter, total length
33.0 cm) |
100 mM borate buffer pH
8.55, 64 mM sodium cholate,
6.1 %v/v n-butanol, 12 mM
γ-cyclodextrin; voltage, 27 kV;
temperature, 21°C |
UV at 220 nm |
CAP (2.40-4.80 mg/
mL)
HCT (1.20-2.40 mg/
mL) |
----- |
[50] |
| CAP & HCT |
Human serum
albumin |
Uncoated fused silica
capillary (35 cm x 50
m ID with 26.5 cm
effective length) |
67 mM phosphate buffer, pH
7.4, I = 0.17, 37 ?C |
UV at 210 nm |
CAP (5-100 μg/mL) |
----- |
[51] |
| CAP |
Human urine and
pharmaceutical
preparations |
Fused-silica capillaries
with a total
length of 57 cm, a
detection length of 50
cm, and an id of
75 mm were employed. |
20 mM phosphate
buffer adjusted to pH 12.0 |
LIFD at 488 nm |
3.5-6000 ng/mL |
0.5 ng/mL |
[52] |
| CAP |
Tablets |
Fused uncoated silica
capillary of 67.5cm total
and 57.5 cm effective
length and of small (50
μm) internal diameter
(ID) and an outer
diameter (o.d.) of 360
μm . |
20 mM phosphate buffer
adjusted to pH 7.0 |
UV at 214 nm |
5-70 μg/mL |
1.5 μg/mL |
[53] |
CAP and its
degradation
products |
Tablets |
Fused-silica capillary 60
cm in total length (52.5
cm to
the detector) and 75 mm
internal diameter (ID).
|
0.025 mM
cetyltrimethylammonium
bromide (CTAB)
added to a sodium phosphate
buffer (pH 5.5; 100 mM) |
UV at 214 nm |
10-80 mg/mL (purity
control) & 80-400
mg/mL (quantitative
determination) |
0.15% |
[54] |
CAP and
Indapamide |
Tablets and
Human Plasma |
50.2 cm long x 50 μm
ID fused-silica capillary |
100 mM borate at pH 9.0 |
UV at 220 nm |
1-100 mg/L |
0.075
mg/L |
[55] |
CAP, lisinopril,
perindoprilat,
quinaprilat and
benazeprilat |
----- |
Uncoated fused-silica
capillaries of 31.2
cm (21 cm from the
injection side to the
detector)375 mm ID |
150 mM HEPES
(2-[4-(2-hydroxyethyl)-1-
piperazine] ethane sulfonic
acid) adjusted with 1 M NaOH
to pH 8.0 at 37?C |
UV at 230 nm |
----- |
----- |
[56] |
HCT and
Telmisartan |
Pharmaceutical
preparations |
Uncoated fused-silica
capillary of 38 cm length
(30 cm effective length)
x 50 μm ID |
25 mM phosphate buffer at pH
2.50 (CZE method) |
UV at 230 nm |
0.010-0.500 mg/mL |
0.008
mg/mL |
[57] |
HCT and
Telmisartan |
Pharmaceutical
preparations |
Uncoated
fused-silica capillary
of 38 cm length (30 cm
effective length) x 50
μm ID |
50 mM borate buffer at pH 9.50
containing 25 mM sodium
dodecyl sulfate as surfactant
(MEKC method) |
UV at 230 nm |
0.010-0.500 mg/m L |
0.062 mg/
mL |
[57] |
HCT and
Carvedilol |
Tablets |
Fused silica capillary
(55 cmx75 μm id) |
Phosphate buffer (12.5 mM, pH
7.4)-methanol (95+5, v/v) |
UV at 226 nm |
0.2-150 μg/mL |
0.07 μg/
mL |
[58] |
HCT and
Metoprolol
|
Tablets |
50.2-cm long x 50
μmIDfused-silica
capillary |
UV at 214 nm |
2.5-250 μg/mL |
0 0.01 μg/
mL |
0 0.01 μg/
mL |
[59] |
HCT,
chlorothiazide,
salamide, and
Zofenopril |
Tablets |
Uncoated fused-silica
capillary (50 μm ID
x 48.5 cm and 40 cm
effective length) |
Sodium borate (pH 9.15; 10
mM) |
UV at 225.0 nm |
10.0-100.0 μg/mL |
2.78 μg/
mL |
[60] |
HCT, Valsartan
and Amlodipine
besylate |
Tablets |
Fused-silica capillary of
57.0-cm-long (50.0-cm
effective length) and
75.6m ID |
40 mM phosphate buffer at
pH 7.5 |
UV at 230 nm |
2-20 μg/mL |
0.65 μg/
mL |
[61] |
HCT, Benazepril
and Amlodipine
besylate |
Tablets |
Fused silica capillary
(78.5 cm total length,
70 cm effective length,
and 75μm ID) |
40 mM phosphate buffer at
pH 7.5 |
UV at 225nm |
10-80μg/mL |
1.224 μg/
mL |
[62] |
HCT, enalapril,
lisinopril,
quinapril,
fosinopril,
ramipril, and
cilazapril |
Tablets |
Fused-silica capillary
52 cm total length (44.5
cm to the detector) with
an internal diameter of
75 mm.
|
Sodium phosphate buffer (pH
7.25; 100 mM). |
UV at 214 nm |
0.016-0.200 mg/ml
(Enalapril maleate &
HCT) |
---- |
[63] |
0.020-0.400 mg/ml
(Lisinopril dihydrate
& HCT) (Quinapril.
HCl & HCT) (Ramipril
& HCT) (Cilazapril &
HCT) |
|
[63] |
0.010-0.200 mg/ml
(Fosinopril sodium
&HCT) |
HCT, candesartan,
eprosartan
mesylate,
irbesartan, losartan
potassium,
telmisartan, and
valsartan. |
Tablets |
Fused-silica capillary
was used, 85 cm in total
length (33 cm to the
detector), and 50 mm
internal diameter (ID) |
60 mM sodium phosphate
buffer pH 2.5 (CZE method) |
UV at 214 nm |
0.04-0.20 mg/ml
(Irbesartan & HCT) |
----- |
[64] |
0.03-0.15 mg/ml
(Losartan potassium
& HCT) |
HCT, candesartan,
eprosartan
mesylate,
irbesartan, losartan
potassium,
telmisartan, and
valsartan. |
Tablets |
Fused-silica capillary
was used, 85 cm in total
length (33 cm to the
detector), and 50 mm
internal diameter (ID) |
55 mM sodium phosphate
buffer pH 6.5 containing 15
mM SDS (MEKC method) |
UV at 214 nm |
0.02-0.10 mg/ml
(Losartan potassium
& HCT) |
----- |
[64] |
0.05-0.25 mg/ml
(Valsartan & HCT) |
losartan with
chlorthalidone or
HCT |
Capsules |
Fused-silica capillaries
coated with polyacrylate
48.5 cm (40 cm effective
length) 75 μm ID 375
μm O.D. |
50 mmol/L-1 of sodium
carbonate buffer at pH 10.3 |
UV at 226 nm |
----- |
0.07980
mg |
[65] |
2. Chromatographic methods
2.1. HPLC methods
| Drugs |
Matrix |
Column |
Mobile phase |
Detector |
Linearity range |
LOD |
Ref |
CAP and
cimetidine |
Tablet |
Purospher star
C18 (5μm, 25 x
0.46 cm) |
Methanol: water (60:40 v/v) |
UV at 225 nm |
9.3 - 150
μg/mL |
1.75 ng/mL |
[66] |
| CAP |
Human plasma |
C18 column
(5 μm, 150 mm x
4.6 mm) |
Methanol (75%, v/v) and
phosphate buffer (25%,
pH = 8, 0.01 M |
UV at 290 nm |
3-2000
ng/mL |
0.9 ng/mL |
[67] |
| CAP & HCT |
Human urine |
Zorbax C8
column |
0.05M sodium acetate,
acetonitrile, methanol
(14:17:4; pH6.5) |
UV at 254nm |
CAP (8 - 160ng)
HCT (6 -140 ng) |
3 and 2 ng
for both |
[68] |
| CAP |
Pharmaceutical
dosage forms |
Luna C18 column
at 50 °C |
Phosphoric acid 15 mm and
acetonitrile |
UV at 210 nm |
5.05-50.5 μg/mL |
1,130 μg/mL |
[69] |
| CAP |
Tablets |
Zorbax SB-C8
Solvent Saver
Plus (3 x 100
mm, 3.5 μm) |
Phosphoric acid (c = 15
mmol) in water-acetonitrile
(w = 60-40 %), |
UV at 260 nm |
12-100 μg/mL |
0.1 μg/mL |
[70] |
| CAP & HCT |
Tablets |
Beckman
Ultrasphere ODS
(4.6 mm x 15 cm,
5μm) |
Methanol/water (45:55
v/v). The pH 3.8 with 85%
orthophosphoric acid
|
UV at 210 nm |
CAP (0.02-0.2
mg/mL)
HCT (0.01-0.1
mg/mL) |
CAP
(5 μg/mL)
HCT
(2 μg/mL)
|
[71] |
| HCT |
Human plasma |
Shim-pack
cyanopropyl
column (250 x
4.6 mm, 5 μm) |
10 mm ammonium acetate
solution (pH 6.0)-methanol
(65:35, v/v) |
UV at 270 nm |
0.31-3.12 (μg/
mL) |
0.043
(μg/mL)
|
[72] |
Amlodipine
Besylate;
Valsartan; HCT |
Tablet |
Phenomenex
Kinetex
(150 x 4.6 mm)
|
Acetonetrile-phosphate buffer
(0.05 M) with pH 2.8 ± 0.2
(40/60, v/v) |
UV at 227 nm |
1-12 μg/mL |
0.39 μg/mL |
[73] |
| CAP |
Plasma sample |
Hypersil BDS C8
(250 X 4.6 mm) |
Phosphate buffer: acetonitrile
(75:25 v/v) pH adjusted at
2.8 with o-phosphoric acid
|
UV at 205 nm |
50-2.000 ng/mL. |
1.65 ng/mL |
[74] |
Enalapril maleate
and HCT |
Tablet |
Li Chrosorb
RP-18 (250 x 4.6
mm, 10 μm) |
0.02 M phosphate buffer (pH
3.0)-acetonitrile (50: 50 v/v) |
UV at 225 and
233 nm |
0.5-30 ng/mL |
50 ng/mL |
[75] |
| CAP |
Bulk material,
pharmaceutical
formulation and
serum |
Purospher Start
C18 (250cm x
4.6mm, 5μm)
and Hypersil
ODS C18 (150x
4.6mm, 5 μm) |
Methanol-water 50:50(v/v)
pH 3.0 adjusted by
phosphoric acid |
UV at 215, 220,
225 nm |
1.25-50 μg/mL |
2.0 ng/mL |
[76] |
| CAP |
Plasma |
μbondapak NH2
column (300x3.9
mm) |
Isocratic consisting of
n-hexane-2-propanol-
methanol-acetic acid
(68:15:15:2). |
UV at 246 nm |
12.5-500 ng/ml. |
3.03 ng/ml. |
[77] |
| CAP and Statins |
Pharmaceutical
preparations and
human serum |
Purospher Star
C18 (5mm, 250 x
?4.6 mm) |
Acetonitrile:water (60:40
v/v) adjusting pH to 2.9. |
UV at 230 nm |
2.5-100 μg/mL |
2.3 ng/mL |
[78] |
Carvedilol and
HCT |
Tablet |
Zorbax SB-C8
column (4.6 x
250 mm, 5 μm) |
0.025 M phosphoric acid and
acetonitrile |
UV at 271 nm |
5-200 μg/mL |
0.30 μg/mL |
[79] |
Amlodipine
Besylate,
Valsartan, and
HCT |
Tablet |
Phenomenex
Luna C18 column
- RP 150 mm x
4.6 mm, 5-μm) |
Acetonitrile :methanol:50 mm
phosphate buffer adjusted to
pH 3 with orthophosphoric
acid |
UV at 239 nm |
1-10 μg/mL |
0.1636 μg/mL |
[80] |
| CAP & HCT |
Human plasma |
DIAMONSIL
C18 column
(150 mm x 4
mm, 5 μm) |
Acetonitrile-trifluoroacetic
acid-water gradient elution
|
UV at 263nm |
CAP
(20-4000 ng/mL)
HCT
(10-1200 ng/mL) |
CAP
(7 ng/mL)
HCT
(3.3 ng/mL) |
[81]
|
| CAP |
Human plasma |
Spherisorb C18
column (250 x 4
.6mm) |
Water:acetonitrile: acetic acid
mixture (44:55:0.2, v/v/v) |
UV at 258 nm |
5-500 ng/mL |
2 ng/mL |
[82] |
| HCT |
Pharmaceutical
Formulations and
Biological Fluid |
ODS Hypersil
C18 (250 mmx4.6
mm, 5 μm) |
Acetonitrile (10.6%),
methanol (16.2%), |
UV at 210 nm |
1.25-12.75 μg/
mL |
1.09 μg/mL |
[83]
|
HCT, amlodipine,
and losartan |
Tablet |
phenomenex luna
5μ CN 100R, 250
x 4.60 mm, 5
micron |
Acetonitrile, water and 0.4%
of potassium dihydrogen
phosphate buffer pH 2.7
adjusted with orthophosphoric
acid (45:35:20). |
UV at 230 nm |
12.5-62.5 μg/mL |
0.03 μg/mL |
[84] |
Zofenopril and
HCT |
Tablets |
Agilent
LiChrospher C18
column (250 x
4.0mm, 5μm) |
Water-TFA (99.9:0.1 v/v)
and (B) acetonitrile-TFA
(99.1:0.1 v/v) |
UV at 224nm |
1.0-20 μg/mL |
0.019 μg/mL |
[85] |
| CAP |
Rabbit plasma |
ODSI C18 (250
mm x 4.6 mm,
5 μm) |
Water: acetonitrile (60:40
v/v), pH adjusted to 2.5 by
using 85% orthophosphoric
acid |
UV at 203 nm |
3.125-100 μg/
mL |
3.10 ng/mL |
[86] |
| CAP |
Human plasma |
Acquity UPLC
BEH shield RP
(1.7μm, 2.1 x
150 mm)) |
Methanol: water containing
0.1% Formic acid (10: 90 v/v
for 1 min then 95: 5 v/v till
the end of the run) |
MS |
10-2000 ng/mL |
3.03 ng/mL |
[87] |
Bisoprolol and
HCT |
Human plasma |
Purosphere
STAR C8
(125
mm x 4 mm, 5
μm) |
Ammonium acetate solution
(1 mM) with formic acid
(0.2%): methanol and
acetonitrile (65:17.5:17.5,
v/v/v (%)) |
MS-MS/ESI. |
1.00-80.00 ng/
mL |
1.00 ng/mL |
[88] |
| CAP |
Tablets |
Phenomenex
Luna 5 μm (C18)
column |
Phosphate buffer (adjusted to
pH 3.0): acetonitrile in a ratio
of 70:30 (v/v) |
ESA Coulometric
detector at 300 |
2-70 μg/mL |
0.6 μg/mL |
[89] |
| CAP |
Blood samples |
a Genesis C8
column, (150 mm
x 4.6 mm) |
Acetonitrile (70%), water
(30%) and trifluoroacetic acid
(0.1%), |
MS-MS /EIS |
2 - 4000 ng/mL |
0.6 ng/mL |
[90] |
| CAP |
Dried blood spot
samples |
Zorbax Eclipse
Plus C8
column
(150 mm x 3.0
mm, 3.5 μm) |
Acetonitrile containing 0.1%
v/v formic acid (eluent A),
water containing 0.1% v/v
formic acid (eluent B) and
isopropanol (eluent C). This
was delivered at 0.5 ml/min
with gradient elution. |
HRMS |
10-400 ng/ml |
----- |
[91] |
Irbesartan and
HCT |
Human plasma |
Acquity U-HPLC
BEH C18 column |
A gradient mpbile phase
with solvent A (0.1% formic
acid in water) and solvent B
(acetonitrile) |
MS-MS/ESI |
0.5-300 ng/mL |
0.15 ng/mL |
[92] |
| HCT |
Human plasma |
Onix C18
Monolitic column
(Phe- nomenex,
(50 x 4,6 mm)
|
Acetonitrile and water (80:20,
v/v), add 5% Isopropyl
alcohol |
MS-MS/ESI. |
5-400 ng/mL |
1.15 ng/mL |
[93] |
Triamterene and
HCT |
Human plasma |
Zorbax Eclipse
Plus RRHD C18
column
(2.1 mmx50
mm, 1.7 μm) |
0.1% formic
acid:methanol:acetonitrile
5:4:1 and 0.1% formic acid
in water at a flow rate of 0.4
ml/min |
MS |
2.5-400 ng/mL |
0.75 ng/mL |
[94] |
2.2. HPTLC methods
| Drugs |
Matrix |
Stationary phase |
Mobile phase |
Detector |
Linearity range |
LOD |
Ref |
| CAP |
Tablets |
Silica gel,
chromatographic
plates 60 F254
?Merck?, and
?Sorbfil? |
Cloroform
R-propanol R
(9:1) |
UV at 254 nm |
----- |
0,4 μg |
[95]
|
| CAP |
Tablets |
Precoated silica gel
60 F |
Methanol: ethyl
acetate: glacial
acetic acid (5:
5: 0.5, v/v/v) |
UV at 241 nm |
6-30 μg/band |
0.022 μg |
[96] |
| lisinopril and
HCT |
Pharmaceutical
tablets.
|
Merck HPTLC
aluminum plates of
silica gel 60 F254, |
Chloroform-
ethylacetate-
acetic acid
(10:3:2, v/v/v) |
UV absorption
and first
derivative
spectra of the
mixture. 210
and 275 nm |
----- |
----- |
[97] |
| Valsartan and
HCT |
Tablet Dosage
Form |
Precoated silica gel
60 F(254) |
Chloroform:
methanol:
toluene: glacial
acetic acid
(6:2:1:0.1,
v/v/v/v) |
UV at 260 nm |
100 - 600 ng/
spot |
30 and 100 ng/
spot |
[98] |
3. Spectroscopic methods
3.1. Spectrophotometric methods
| Drugs |
Matrix |
Method-reagent |
? max (nm) |
Linearity range |
LOD |
Ref |
| Enalapril, HCT and walsartan |
Complex
pharmaceutical
preparations |
Derivative
spectrophotometry |
----- |
Linearity range |
LOD |
Ref. |
| Triamterene and HCT |
Tablets |
Zero-crossing technique |
255.7 and 283.2 |
1.25- 6.25 μg/mL |
0.25 μg/mL |
[100] |
| Metoprolol and HCT |
Pharmaceutical
preparations |
Zero-crossing |
282 |
12.5 - 37.5 μg/mL |
1.5 μg/mL |
[101] |
HCT, Atenolol and Losartan
potassium
|
Tablet |
Simultaneous equation
method
First order derivative
method |
272.5, 224 and 250
280.5, 233 and 244 |
|
|
|
| Carvedilol and HCT |
Combined dosage
form |
Dual wavelength
analysis |
266 and 289.4 |
----- |
----- |
[103]
|
Olmesartan medoxamil,
amlodipine besylate and HCT |
Tablets |
Ratio subtraction
method |
315 |
2-40 μg/mL |
0.819 μg/mL |
[104] |
Olmesartan medoxomil and
HCT |
Tablet |
Absorption ratio
spectrophotometric
method |
272.8 |
10-30 μg/mL |
0.44 μg/mL |
[105] |
HCT, indapamide and
xipamide |
Pharmaceutical
tablets |
Ternary complex
formation with
eosin and lead (II)
in the presence of
methylcellulose as
surfactant |
543 |
8-40 μg/mL |
----- |
[106] |
HCT and Olmesartan
Medoxomil |
Combined dosage
form |
UV spectrophotometric
method |
271.5 and 257 |
5-25 μg/mL |
----- |
[107] |
HCT and amiloride
hydrochloride |
Pharmaceutical
dosage forms |
Isoabsorptive point |
274.7 |
10-80 μg/mL |
0.39 μg/mL |
[108] |
| HCT and telmisartan |
Tablet dosage form. |
Simultaneous equation
method |
258 and 299 |
2-20 μg/mL |
0.079 μg/mL |
[109]
|
3.2. Spectrofluorimetric methods:
| Drugs |
Matrix |
Fluorogenic reagent (method) |
?ex (nm) |
?em (nm) |
Linearity
range |
LOD |
Ref |
| CAP |
Tablets |
Cerium (IV) in the presence of
sulphuric acid |
256 |
354 |
0.1-1.3 μg/
mL |
0.016 μg/mL |
[110] |
| HCT and
timolol |
Tablets |
-------- |
270 |
375 |
4-12 μg/mL |
0.0104 mg/L |
[111]
|
| HCT and
TELM |
Tablets |
1 M sodium hydroxide |
230 |
365 |
50-400 ng/
mL |
------ |
[112] |
| HCT |
Tablets |
Carbon dot via inner filter effect
(IFE) and resonance Rayleigh
scattering (RRS) |
310 |
434 |
0.17-2.50 μg/
mL |
0.11 μg/mL |
[113] |
| HCT |
Tablets |
Acetonitrile at ph 6.2 and Tb3+ ion
doped in sol-gel matrix |
370 |
545 |
5.0x10-10
- 5.0x10-6
mol/L |
2.2x10-11
mol/L |
[114] |
4. Electrochemical methods:
| Drug |
Matrix |
Electrode |
Linearity range |
LOD |
Ref. |
| CAP & HCT |
Tablet and Urine |
Graphene/ferrocene composite carbon
paste (GR/Fc/CP) |
CPT (1.0-430 μM)
HCT (0.5-390 μM) |
------ |
[115] |
| CAP |
Tablet |
Platinum electrode in a 0.1 M HNO3
solution at 1.2 V versus a saturated
silver-silver chloride |
1.2x10-6 - 3.2x10-4
M |
9.2x10-7 M |
[116] |
| CAP & HCT |
Tablet and Urine |
Carbon ionic liquid modified with
copper hydroxide nanoparticles |
CPT (0.7-70 μM)
HCT (3-600 μM) |
12 nM
60 nM |
[117] |
| CAP |
Urine |
Zinc oxide nanoparticles and a new
ferrocene-derivative modified carbon
paste |
0.09-450.0 μmol/L |
0.05 μmol/L |
[118] |
| CAP |
Urine |
Ferrocene-dicarboxylic acid modified
carbon paste |
3.0x10-7 - 1.4x10-4 M |
9.1x10-8 M |
[119] |
| CAP |
Urine |
Catechol-derivative-multiwall carbon
nanotubes paste |
6.4x10-8 - 3.2x10-48
mol/L |
3.4x10-8 mol/L |
[120]
|
CAP, acetaminophen,
tyrosine and HCT
|
Tablet and Urine |
Nio/cnts and (2-(3,
4-dihydroxyphenethyl) isoindoline-1,
3-dione) (DPID). |
CAP (0.07-200.0
μM)
HCT (10.0-600.0
μM) |
9.0 nM
5.0 μM |
[121] |
| HCT |
Tablet and Urine |
Glassy carbon |
24-320 ng/mL |
5.0 ng/mL |
[122]
|
| CAP |
Serum and
pharmaceutical
formulations |
A three-electrode system containing the
static mercury drop electrode (SMDE),
Pt auxiliary electrode and Ag/agcl
reference electrode was used throughout |
0.5-50.0 μg/mL |
6.28x10-3 μg/mL |
[123]
|
| CAP |
Urine |
Amalgam film (Hg(Ag)FE) |
0.05-1 μM |
1.9 nM |
[124] |
| CAP |
Injection |
Boron-doped diamond thin film
electrode |
50 μM - 3 mM |
25 μM |
[125] |
| HCT |
Urine |
Electrochemically pretreated pencil
graphite electrode (EPPGE) using cyclic
voltammetry (CV), differential pulse
voltammetry (DPV) and square wave
voltammetry (SWV) |
DPV (4 μM - 140
μM)
SWV (1 μM - 20 μM) |
3.25 μM/L
0.421 μM/L |
[126] |
| CAP |
Urine |
Manganese supported on an organomodified sio2/Al2O3 |
3.0x10-7 - 300x10-4
mol/dm3 |
9.0x10-8 mol/dm3 |
[127] |
|
Tablet and Urine |
Two dimensional single-crystal
hexagonal gold nanosheets (schgnss)
were prepared by microwave heating of
a solution of haucl4 in an ionic liquid |
2-400 nM and 4.0-50
μM |
0.3 nM |
[128] |
| HCT |
Urine |
Nickel hydroxide |
1.39x10 -5 - 1.67x10-4
mol/L |
7.92x10-6 mol/L |
[129] |
| CAP |
Urine |
Nio nanoparticle modified (9,
10-dihydro-9, 10-ethanoanthracene-11,
12-dicarboximido)-4-ethylbenzene-1,
2-diol carbon paste electrode |
0.035 - 550 μmol/L |
0.007 μmol/L |
[130] |
| Methyldopa and HCT |
Tablet, Urine and
Pill |
A molybdenum (VI) complex-ionic
liquid-zno NP modified carbon paste
electrode (MCILZNMCPE) |
0.05 - 300.0 μM |
------- |
[131]
|
| CAP |
Tablet and Urine |
Multiwall Carbon Nanotubes
Paste Electrode in the Presence of
Isoproterenol as a Mediator |
0.3 - 90 μmol/L |
0.1 μmol/L |
[132] |
| CAP |
|
Glassy carbon in the presence of 4,
4'-biphenol |
25-300 μM |
3.34 μM |
[133] |
CAP, acetaminophen,
tryptophan, folic
acid, and L-cysteine |
Urine and Plasma |
A novel carbon paste electrode
(CPE) modified with 2,2'-[1,7-hepta
nediylbis(nitrilomethylidene)]-bis(4-
hydroxyphenol) (DHB) and carbon
nanotubes (cnts) |
7.0-100.0 and
100.0-2,500.0 μM |
2.43 μM |
[134] |
| HCT |
Tablets |
A Trypan Blue modified combined
pencil graphite electrode system (tybGGG) |
DPV (0.5-7 μM)
SWV (0.1-5 μM) |
0.1327 μM
0.0320 μM |
[135] |
| metoprolol and HCT |
Urine |
Cathodically pretreated boron-doped
diamond (BDD) |
0.51-18.7 μmol/L |
0.376 μmol/L |
[136] |
Conclusion
This literature review represents an up-to-date survey about
pharmacological action and all reported methods that have been
developed for determination of captopril and hydrochlorothiazidein
their pure form, combined form with other drugs, combined form
with degradation products, and in biological samples such as
electrophoresis, liquid chromatography, spectrophotometry,
spectroflourimetry, voltammetry, etc.
References
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green
LA, et al. (2003) National Heart, Lung, and Blood Institute Joint
National Committee on Prevention, Detection, Evaluation,
and Treatment of High Blood Pressure, & National High
Blood Pressure Education Program Coordinating Committee.
The Seventh Report of the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High
Blood Pressure: the JNC 7 report. JAMA 289: 2560-2572.
- Chobanian AV, Bakris G L, Black H R, Cushman WC, Green
LA, Izzo et al. (2003) Joint National Committee on Prevention,
Detection, Evaluation, and Treatment of High Blood Pressure.
National Heart, Lung, and Blood Institute, & National High
Blood Pressure Education Program Coordinating Committee.
Seventh report of the Joint National Committee on Prevention,
Detection, Evaluation, and Treatment of High Blood Pressure.
Hypertension., (Dallas, Tex.: 1979) 42: 1206-1252.
- Messerli FH, Williams B, Ritz E (2007) Essential hypertension.
Lancet (London, England) 370: 591-603.
- Bramah N Singh, Victor J Dzau, Paul M Vanhoutte,
Raymond L Woosley, DiBianco R, et al. (1994) Book Review
Cardiovascular Pharmacology and Therapeutics. 1231 pp.
New York, Churchill Livingstone, 0-443-8814-4. New
England J. Med 331: 1387-1387.
- Blowey DL (2016) Diuretics in the treatment of hypertension.
Ped.Nephrol., (Berlin, Germany) 31: 2223-2233.
- Plosker G L, McTavish D (1995) Captopril. A review of
its pharmacology and therapeutic efficacy after myocardial
infarction and in ischaemic heart disease. Drugs & aging 7:
226-253.
- Brown NJ, Vaughan D E (1998) Angiotensin-converting
enzyme inhibitors. Circulation 97: 1411-1420.
- Tigerstedt R, Bergman P (1898) Niere und Kreislauf1.
SkandinavischesArchivFurPhysiologie 8: 223-271.
- Goldblatt H, Lynch J, Hanzal RF, Summerville WW (1934)
Studies on Experimental Hypertension : The production of
persistent elevation of systolic blood pressure by means of
renal ischemia. J.exper.Med 59: 347-379.
- Brogden RN, Todd PA, Sorkin EM (1988) Captopril. An
update of its pharmacodynamic and pharmacokinetic
properties, and therapeutic use in hypertension and congestive
heart failure. Drugs 36: 540-600.
- Tamargo J Segura J, Ruilope LM (2014) Diuretics in the
treatment of hypertension. Part 1: thiazide and thiazide-like
diuretics. Expert opin.Pharmacother 15: 527-547.
- Tamargo J Segura J, Ruilope LM (2014) Diuretics in
the treatment of hypertension. Part 2: loop diuretics and
potassium-sparing agents. Expert opin.Pharmacother 15:
605-621.
- Tarazi RC, Dustan HP, Frohlich ED (1970) Long-term thiazide
therapy in essential hypertension. Evidence for persistent
alteration in plasma volume and renin activity. Circulation
41: 709-717.
- Van Brummelen P, Man in 't Veld A J, Schalekamp M A (1980)
Hemodynamic changes during long-term thiazide treatment
of essential hypertension in responders and nonresponders.
Clin. Pharmacol.Ther 27: 328-336.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A,
et al. (2014) Task Force for the Management of Arterial
Hypertension of the European Society of Hypertension and
the European Society of Cardiology 2013 ESH/ESC Practice
Guidelines for the Management of Arterial Hypertension.
Blood pressure 23: 3-16.
- Roush GC, Ernst ME, Kostis JB, Kaur R, Sica DA (2015)
Not just chlorthalidone: evidence-based, single tablet, diuretic
alternatives to hydrochlorothiazide for hypertension. Curr.
hypertension reports 17: 540.
- Roush GC, Ernst ME, Kostis JB, Tandon S, Sica DA
(2015) Head-to-head comparisons of hydrochlorothiazide
with indapamide and chlorthalidone: antihypertensive and
metabolic effects. Hypertension (Dallas, Tex.: 1979) 65:
1041-1046.
- Dineva S Uzunova K, Pavlova V, Filipova E, Kalinov K,Vekov T (2021) Network meta-analysis of efficacy and safety
of chlorthalidone and hydrochlorothiazide in hypertensive
patients. Blood pressure monitor 26: 160-168.
- LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE
(2000) Low-dose hydrochlorothiazide and preservation of
bone mineral density in older adults. A randomized, doubleblind, placebo-controlled trial. Annals of internalmedi 133:
516-526.
- Sebaiy MM, Abdelazeem AI, AboulfotouhA, RoukA
A, Mohamed AA, et al. (2022) Instrumental Analysis of
Chloroquine and Hydroxychloroquine in Different Matrices.
Curr. Res: Integr. Med 7: 1-8.
- Batakoushy HA, Omar MA, Ahmed HM, Abdel Hamid
MA, Sebaiy MM (2022) Review article: Pharmacology
and Analytical Chemistry Profile of Dapagliflozin,
EmpagliflozinandSaxagliptin. Modern App. Pharm.
Pharmacol 2: MAPP 000548.
- Ali OT, Elgendy KM, Saad MZ, Hassan WS, Sebaiy
MM (2021) Analytical Techniques for Determination of
Albendazole, Fenbendazole, Omeprazole and Fluconazole
in Pharmaceutical and Biological Samples. Int. J. Pathol.
Immunol 2: 1-24.
- Lashine EM, El-Sayed AS, ElshahatAK, Zaki AR, ElHalaby AS, et al. (2021) Review article: Pharmacological
and Analytical Profile of Celecoxib. Pharm. Sci. Biomed.
Anal. J 4: 128.
- Ramadan A., Abd-Elaziz A., Ismail E. M., Maher A., Hegazy
K. M., Sebaiy M. M. (2021) Review article: Pharmacological
and Analytical Profile of Celecoxib. Pharm. Sci. Biomed. Anal.
J., 4(1):128 https://scientificliterature.org/Pharmaceutics/
Pharmaceutics-21-128.pdf
- Elsabbagh OI, Soror AW, Moselhy AY, Elayat AE, Hafez AM,
Saleh et al. (2021) Literature Review on Obesity: Causes,
Treatment and Correlation with Pandemic COVID-19. Pharm.
Drug Regul. Affair J. (PDRAJ) 4: 000124.
- Ibrahim SM, Elshafiey EH, Al batreek EK, Abdulrahim ER,
Azazy ER, et al. (2021) Steroids in Medicinal Chemistry:
Literature Review. Academic J. Chem 6: 69-78.
- Ibrahim AE, Elhenawee M, Saleh H, Sebaiy MM (2021)
Overview on Liquid Chromatography and its Greener
Chemistry Application. Ann. Adv. Chem 5: 004-012.
- Abdel-Aziz LM, Soror AA, Hassan AA, Ali AS, Hafez
AA, et al. (2021) Review article: Instrumental Analysis
of Chlordiazepoxide in Different Matrices. Int. Res. J.
Multidiscipl. Technovat. (IRJMT) 3: 1-10.
- Ibrahim AE, ElhenaweeM, Saleh H, Sebaiy MM (2021) Minireview on Glaucoma Drugs, Timolol and Latanoprost: Mode
of Action and Analytical Methods. Open J. Pharm. Sci 1: 1-3.
- Saraya RE, Elhenawee M, Saleh H, Sebaiy MM (2021)
Analytical Review : Methods of Determination for Ledipasvir
and Velpatasvir in Pharmaceutical and Biological samples.
Int. J. Pharm. Sci. Clin. Res. (IJPSCR) 1: 111-118.
- Ibrahim AE, Elhenawee M, Saleh H, Sebaiy MM (2021)
Erectile Dysfunction and Premature Ejaculation Drugs: Mode
of Action and Analytical Methods Literature Review. J. Drug.
Res. Develop 7: 1-7.
- Sebaiy MM, Shanab AG, Nasr AK, Hosney AE, ElsaidAG,
et al. (2021) Literature Review on Spectrophotometric,
Chromatographic and Voltammetric Analysis of Ivermectin.
Med. Anal. Chem. Int. J. (MACIJ) 5: 000170.
- Saraya RE, Elhenawee M, Saleh H, Sebaiy MM (2021)
Review article: Instrumental Analysis of Sofosbuvir and
Daclatasvir in Different Matrices.Innovat Int. J. Med. Pharm
Sci 6: 1-8
- Abdel-Aziz LM, Sapah AA, Naser A, Abd-elazizA, ElEmaryA, et al. (2021) Review article: Spectroscopic,
Chromatographic and Electrochemical Determination of
Indomethacin in Different Matrices. Eur. J. Sci. Innovat.
Technol. (EJSIT) 1: 32-40.
- Sebaiy M. M.,Farouk E. M., LotfyE. M., Mokhtar E. M.,
Abd-ElgwadE. N., et al. (2021) Review article: Spectroscopic,
Chromatographic and Electrochemical Analysis of
Azithromycin in Different Matrices. J. Drug Des. Res 8: 1084.
- Ibrahim AE, ElhenaweeM, Saleh H, Sebaiy MM (2021)
Overview on Hepatitis C, Treatment Strategy, Instrumental
Analysis of Anti-HCV drugs. Pharm. Drug Innovat 2: 1-8.
- Saraya RE, Elhenawee M, Saleh H, Sebaiy MM (2021) Mini
Review: Insights on Instrumental Analysis of Ombitasvir,
Paritaprevir and Ritonavir. Int. J. Chem. Res 5: 1-4.
- Elrefay H, Ismaiel OA, Hassan WS, Shalaby A, Fouad A, et
al. (2021) Mini-Review on Various Analytical Methods for
Determination of Certain Preservatives in Different Matrices.
Int. J. Res. Stud. Sci., Eng. Technol. (IJRSSET) 8: 1-8.
- Ibrahim AE, Elhenawee M, Saleh H, Sebaiy MM (2021)
Mini-review on Chromatography of Proteomics. Glob. J
Chem. Sci 1: 1-4.
- Ali OT, Elgendy KM, Saad MZ, Hassan WS, Sebaiy MM
(2021) Review Article: Instrumental Analysis of Certain
Azoles with Variant Anti-Infective Activity. Pharma Pages
1: 1-15.
- Saraya R E, Elhenawee M, Saleh H, Sebaiy MM (2021)
Review article on Analytical Techniques of Lamivudine
Determination in Different Matrices.J. Adv. Pharm. Sci. Tech
(JAPST) 2: 37-46.
- El-didamoony MA, Elsadek ME, Baraka MM, Ibrahim SM,
Sebaiy MM (2021) Review article: Analytical Methods for
Determination of Certain Antihypertensive Drugs. Biomed.
J. Sci. Tech. Res 34: 26511-26527.
- Saraya RE, Elhenawee M, Saleh H, Sebaiy MM (2021)
Review article: Analytical Methods for Determination of
Ondansetron hydrochloride and Pantoprazole. J. Med. Res.
Health Sci. (JMRHS) 4: 1175-1181.
- Elbaramawi SS, El-Sadek ME, Baraka MM, Abdel-Aziz LM,
Sebaiy MM (2020) Review article: Instrumental Analysis of
Some Anti-ulcer Drugs in Different Matrices. Chem. Reports
2: 156-172.
- Elkady YM, El-AdlS M, Baraka MM, Sebaiy MM (2020)
Literature Review of Analytical Methods for Determination
of Triamcinolone Acetonide and Benzyl Alcohol. Nov. Appro.
Drug Des. Dev 5: 555663.
- Elkady YM, El-Adl SM, Baraka MM, Sebaiy MM (2020)
Review article: Analytical Methods for Determination
of Salbutamol, Ambroxol and Fexofenadine. J. Biotech.
Bioprocess 1: 1-11.
- Ibrahim F, El-Adl SM, Baraka MM, Ibrahim SM, Sebaiy
MM (2020) Review Article: Analytical methods for the
determination of certain antibiotics used in critically ill
patients. J. Pharm. Biopharm. Res 2: 99-117.
- Sebaiy MM, Abdellatef HE, Elhenawee MM, Elmosallamy
MA, Alshuwaili MKh )2020) Review Article: Instrumental
Analysis of Olopatadine Hydrochloride, Oxeladine Citrate,
Amlodipine Besylate and Xipamide. Int. J. Anal. Bioanal.
Methods 2: 10.
- Sebaiy MM, El-Adl SM, Baraka MM, Hassan AA (2020)
Review article: Analytical Methods for Determination of
Certain Sartans and Diuretics. J. Chem. Sci. Chem Eng 1: 1-8.
- Pasquini B, Orlandini S, Caprini C, Del Bubba M, Innocenti
M, et al. (2016) Cyclodextrin- and solvent-modified micellar
electrokinetic chromatography for the determination of
captopril, hydrochlorothiazide and their impurities: A Qualityby Design approach. Talanta 160: 332-339.
- Liu TT, Xiang LL, Wang JL, Chen DY (2015) Application
of capillary electrophoresis-frontal analysis for comparative
evaluation of the binding interaction of captopril with
human serum albumin in the absence and presence of
hydrochlorothiazide. J pharm.Biomed Anal 115: 31-35.
- Perez-Ruiz T, Martínez-Lozano C, Galera R (2006)
Development and validation of a capillary electrophoresis
method with laser-induced fluorescence detection for the
determination of captopril in human urine and pharmaceutical
preparations. Electrophoresis 27: 2310-2316.
- Mukozhiwa SY, Khamanga SMM, Walker RB (2017) the use
of experimental design for the development of a capillary
zone electrophoresis method for the quantitation of captopril.
Pharmazie 72: 518-524.
- Hillaert S, Van den Bossche W (1999) Determination
of captopril and its degradation products by capillary
electrophoresis. J. pharm. Biomed. Anal 21: 65-73.
- Alnajjar AO (2008) Simultaneous CE determination of
captopril and indapamide in pharmaceuticals and human
plasma. Chromatographia 68: 437-442.
- Van Dyck S, Novakova S, Van Schepdael A, Hoogmartens J
(2003) Inhibition study of angiotensin converting enzyme by
capillary electrophoresis after enzymatic reaction at capillary
inlet. J.Chromatogr A 1013: 149-156.
- Stacescu ¸, Hancu G, Gagyi L, SOARE R, Kelemen H (2017)
Simultaneous determination of hydrochlorothiazide and
telmisartan from pharmaceutical preparations using Capillary
Electrophoresis. StudiaUniversitatis Babes-Bolyai, Chemia
62.
- Alzoman NZ, Sultan MA, Maher HM, AlShehri MM, Olah IV
(2013) Validated stability-indicating capillary electrophoresis
method for the separation and determination of a fixed-dose
combination of carvedilol and hydrochlorothiazide in tablets.
J. AOAC Inter 96: 951-959.
- Alnajjar A O, Idris AM, Attimarad M V, Aldughaish A M,
Elgorashe R E (2013) Capillary electrophoresis assay method
for metoprolol and hydrochlorothiazide in their combined
dosage form with multivariate optimization. J. chromatogr
Sci 51: 92-97.
- Fayed AS, Rezk MR, Marzouk HM, Abbas SS
(2018) A capillary zone electrophoresis method with
multiresponsechemometric optimization for the simultaneous
determination of zofenopril calcium and hydrochlorothiazide
in presence of hydrochlorothiazide major impurities. J.
chromatogr. Sci 56: 461-471.
- Ebeid W, Salim M, Elkady E, Elzahr A, El-Bagary R, et al.
(2015) Simultaneous determination of valsartan, amlodipine
besylate and hydrochlorothiazide using capillary zone
electrophoresis (CZE). Die Pharmazie 70: 368-373.
- Ahmed HM, Belal TS, Shaalan RA, El Yazbi FA, Elonsy
SM (2020) Validated capillary zone electrophoretic method
for simultaneous analysis of benazepril in combination
with amlodipine besylate and hydrochlorothiazide.
ActaChromatogr 32: 219-227.
- Hillaert S, De Grauwe K, Van den Bossche W (2001)
Simultaneous determination of hydrochlorothiazide and
several inhibitors of angiotensin-converting enzyme by
capillary electrophoresis. J.Chromatogr A 924: 439-449.
- Hillaert S, Van den Bossche W (2003) Simultaneous
determination of hydrochlorothiazide and several angiotensinII-receptor antagonists by capillary electrophoresis. J. pharm.
Biomed. Anal 31: 329-339.
- Balesteros MR, Faria AF, De Oliveira MA (2007)
Determination of losartan associated with chlorthalidone
or hydrochlorothiazide in capsules by capillary zone
electrophoresis. J. Braz. Chem. Soc 18: 554-558.
- Sultan N, Naveed S, Arayne M S (2013) RP-HPLC Method
for the simultaneous determination of captopril and H2-
receptor antagonist: Application to interaction studies. Med.
Chem 3: 183-187.
- Rastkari N, Khoobi M, Shafiee A, Khoshayand MR,
Ahmadkhaniha R (2013) Development and validation of a
simple and sensitive HPLC-UV method for the determination
of captopril in human plasma using a new derivatizing reagent
2-naphthyl propiolate. J Chromatogr. B 932: 144-151.
- Khedr A, El-Sherief H (1998) 3-bromomethyl-propyphenazone
as a new derivatization reagent for high performance liquid
chromatography of captopril and hydrochlorothiazide with
UV-detection. Biomed.Chromatogr 12: 57-60.
- Carje AG, Balint A, Ion V, Pop AL, Muntean DL, et al. (2019)
HPLC-UV method approach for the analysis and impurity
profiling of captopril. Stud. Univ. Babes-Bolyai Chem 64:
231-242.
- 70. Donath-NagyG,Vancea S, Imre S (2011) Comparative study
of captopril derivatization reaction by LC-UV, LC-MS and
CE-UV methods. CroaticaChemicaActa 84: 423-427.
- Ivanovic D, Medenica M, Malenovic A, Jancic B (2004)
Validation of the RP-HPLC method for analysis of
hydrochlorothiazide and captopril in tablets. Accred.Qual.
Assur 9: 76-81.
- Salama I (2011) Simultaneous HPLC-UV analysis of
telmisartan and hydrochlorothiazide in human plasma. Bull.
Fac. Pharm Cairo Univ 49: 19-24.
- El-Gizawy SM, Abdelmageed OH, Omar MA, Deryea SM,
Abdel-Megied AM (2012) Development and validation of
HPLC method for simultaneous determination of amlodipine,
valsartan, hydrochlorothiazide in dosage form and spiked
human plasma. Amer.J. Anal. Chem 3: 422.
- IQBAL FM, Ahmad M, Zubair MM, Tulain UR, Rashid
A (2015) Determination of captopril in plasma by highperformance liquid chromatography: Application in an invivo evaluation of drug release from hydrogel. Lat. Am. J.
Pharm 34: 875-84.
- Carlucci G, Di Giuseppe E, Mazzeo P (1993) Simultaneous
determination of enalapril maleate and hydrochlorothiazide
in tablets by derivative UV spectrophotometry and highperformance liquid chromatography. Int. J. pharm 93: 245-
248.
- Naveed S, Sultana N, Arayne MS (2013) Method for
the Determination of Captopril in Bulk, Pharmaceutical
Formulations and Serum by HPLC using two different
System. Amer. Based Res J 2.
- Amini M, Zarghi A, Vatanpour H (1999) Sensitive highperformance liquid chromatographic method for determination
of captopril in plasma. Pharma.ActaHelvetiae 73: 303-306.
- Sultana N, Arayne M S, Naveed S (2010) Simultaneous
determination of captopril and statins in API, pharmaceutical
formulations and in human serum by RP-HPLC. J. Chin.
Chem. Soc 57: 378-383.
- Belal TS, Shaalan RA, El Yazbi FA, Elonsy SM (2013)
Validated stability-indicating HPLC-DAD determination
of the antihypertensive binary mixture of carvedilol and
hydrochlorothiazide in tablet dosage forms. Chromatographia
76: 1707-1720.
- Anandakumar K, Jothieswari D, Subathrai R, Santhi DV,
Vetrichelvan T (2012) Validated RP-HPLC method for the
simultaneous determination of amlodipine besylate, valsartan,
and hydrochlorothiazide in bulk and in pharmaceutical
formulation. ActaChromatogr 24: 37-50.
- Huang T, He Z, Yang B, Shao L, Zheng X, et al.
(2006) Simultaneous determination of captopril and
hydrochlorothiazide in human plasma by reverse-phase
HPLC from linear gradient elution. J. Pharm. Biomed. Anal
41: 644-648.
- Li K, Tan L, Zhou JH (1996) HPLC determination of captopril
in human plasma and its pharmacokinetic study. Biomed.
Chromatogr 10: 237-239.
- Haque S M (2022) Box-Behnken Experimental
Design for Optimizing the HPLC method to Determine
Hydrochlorothiazide in Pharmaceutical Formulations and
Biological Fluid. J. Mol. Liq 118708.
- Tengli A R, Gurupadayya B M , Soni N (2013)Simultaneous
estimation of hydrochlorothiazide, amlodipine, and losartan
in tablet dosage form by RP-HPLC. Int.J. chem.Anal.Sci 4:
33-38.
- Carlucci G, Di Federico L, Iuliani P (2010) HPLC-DAD
method for the simultaneous determination of zofenopril and
hydrochlorothiazide in oral pharmaceutical formulations.
J.Sep.Sci 33: 1717-1722.
- Rasool M F, Qureshi U F, Ranjha N M, Imran I, Nisa M U,
et al. (2021) Development and validation of reversed phase
high performance liquid chromatography (RP-HPLC) for
quantification of captopril in rabbit plasma. ActaChromatogr
33: 315-321.
- Elzanfaly ES, Merey HA (2017) A liquid chromatography/
tandem mass spectrometric method for determination of
captopril in human plasma: application to a bioequivalence
study. J. Appl. Pharm. Sci 7: 008-015.
- Tutunji M F, Ibrahim H M, Khabbas M H, Tutunji L F
(2009) Simultaneous determination of bisoprolol and
hydrochlorothiazide in human plasma by HPLC coupled
with tandem mass spectrometry. J. Chromatogr 877: 1689.
- Khamanga S M, Walker R B (2011) The use of experimental
design in the development of an HPLC-ECD method for the
analysis of captopril. Talanta 83: 1037-1049.
- Soares A K A, De Moraes M O, Bezerra F A F, De Nucci
G, De Moraes M E A (2012) Determination of captopril
by hplctandem mass spectrometry: application in a
bioequivalence study. RevistaBrasileiraemPromocao da
Saúde 25: 13-19.
- Lawson G, Mulla H, Tanna S (2012) Captopril Determination
in Dried Blood Spot Samples with LC-MS and LCHRMS:
A Potential Method for Neonate Pharmacokinetic Studies.J.
Bioanal. Biomed 4,2
- Qiu X, Wang Z, Wang B, Zhan H, Pan X, Xu R A
(2014) Simultaneous determination of irbesartan and
hydrochlorothiazide in human plasma by ultra high
performance liquid chromatography tandem mass
spectrometry and its application to a bioequivalence study.
J.Chromatogr 957: 110-115.
- 93. Sousa C E, Bedor D C, Goncalves T M, Ramos V L, Carvalho
A L, et al. (2009) Rapid determination of hydrochlorothiazide
in human plasma by high performance liquid chromatographytandem mass spectrometry. Lat. Amer. J. Pharm 28: 793-797.
- Margaryan T, Mikayelyan A, Zakaryan H, Armoudjian
Y, Alaverdyan H (2019) Simultaneous determination of
Triamterene and Hydrochlorothiazide in human plasma by
liquid chromatography tandem mass spectrometry and its
application to a bioequivalence study. SN Appl. Sci 1.
- 95. Liliya L (2016) Development of methodology for identification
of captopril in medicines. Asian J. Pharm 10.
- Chitlange S S, Soni R, Wankhede S B, Sakarkar D M (2009)
A stability-indicating HPTLC method for estimation of
Captopril in pharmaceutical dosage form. J. Pharm Res 2.
- El-GindyA, Ashour A,Abdel Fattah L,Shabana MM (2001)
Spectrophotometric and HPTLC-densitometric determination
of lisinopril and hydrochlorothiazide in binary mixtures. J.
Pharm. Biomed. Anal 25: 923-931.
- Shah N J, Suhagia B N, Shah R R, Patel N M (2009) HPTLC
Method for the Simultaneous Estimation of Valsartan and
Hydrochlorothiazide in Tablet Dosage Form. Ind.J. pharm.
Sci 71: 72-74.
- Stolarczyk M, Ma?lanka A, Krzek J, Milczarek J (2008)
Application of derivative spectrophotometry for determination
of enalapril, hydrochlorothiazide and walsartan in complex
pharmaceutical preparations. Acta Pol. Pharm 65: 275-281.
- Stolarczyk M, Apola A, Krzek J, Lech K (2008)Simultaneous
determination of triamterene and hydrochlorothiazide in
tablets using derivative spectrophotometry. Acta Pol. Pharm
65: 283-287.
- Stolarczyk M, Ekiert R, Krzek J, Rzeszutko W (2006)
Determination of metoprolol and hydrochlorothiazide by
derivative spectrophotometric method in pharmaceutical
preparations. Acta Pol. Pharm 63: 169-173.
- Thomas AB, Chavan UB, Nanda RK, Kothapalli LP,
Deshpande AD, Jagdale SN, Dighe SB (2009)Simultaneous
spectrophotometric estimation of Hydrochlorothiazide,
Atenolol and Losartan potassium in tablet dosage form.
Hindustan Antibiot Bull 51: 33-38.
- Abdelwahab N S (2016) Spectrophotometric methods
for simultaneous determination of Carvedilol and
Hydrochlorothiazide in combined dosage form. Arab. J.
Chem 9: 355-360.
- Darwish HW (2013) Application of smart spectrophotometric
methods and artificial neural network for the simultaneous
quantitation of olmesartanmedoxamil, amlodipine besylate
and hydrochlorothiazide in their combined pharmaceutical
dosage form. Chem. Cent. J 7: 22.
- Rote AR, Bari PD (2010) Spectrophotometric estimation of
olmesartanmedoxomil and hydrochlorothiazide in tablet. Ind.
J.Pharm. Sci. 72: 111-
- Omar MA (2010) Spectrophotometric and spectrofluorimetric
determination of certain diuretics through ternary complex
formation with eosin and lead (II). J Fluoresc 20: 275-281.
- HemkeAT, BhureM V, Chouhan K S, Gupta K R, WadodkarS
G (2010) UV Spectrophotometric Determination of
Hydrochlorothiazide and OlmesartanMedoxomil in
Pharmaceutical Formulation. J. Chem 7, ID 826585, 6.
- AbdelaleemE A, Naguib I A, ZaazaaH E,DrazM E (2014)
Spectrophotometric Methods for Quantitative Determination
of Binary Mixture of Hydrochlorothiazide and Amiloride
Hydrochloride without Prior Separation. Asian J. Biomed.
Pharm. Sci 04: 27-33.
- Rekha G, Narendra S, Anand G, Mukesh M, Ranjit S
(2011) Spectrophotometric simultaneous determination of
hydrochlorothiazide and telmisartan in combined dosage form
by dual wavelength method. Int. J.Comprehens. Pharm 2: 1-3.
- Eldidamony A (2009) Spectrofluorimetric Determination of
the Hypertensive Drug Captopril Based on Its Oxidation with
Cerium(IV). J. Chin.Chem.l Soc 56, 10.
- Fadel W, Anas L, MakasebM (2020) Simultaneous
spectrofluorometric analysis of tablets containing
hydrochlorothiazide combined with timolol maleate or
amiloride hydrochloride.Ac ta Pharm 70: 373-385.
- Bebawy L I, Abbas S S, Fattah L A, RefaatH H
(2005) Application of first-derivative, ratio derivative
spectrophotometry, TLC-densitometry and spectrofluorimetry for the simultaneous determination of telmisartan and
hydrochlorothiazide in pharmaceutical dosage forms and
plasma, Il Farmaco, 60: 859-867.
- GhafarlooA, Sabzi R E, SamadiaN,HamishehkarbH (2022)
Spectrofluorimetric Determination of Hydrochlorothiazide
by a Carbon Dots-Based Probe via Inner Filtering Effect
and Resonance Rayleigh Scattering. J. Braz. Chem. Soc 33:
361-368.
- Youssef AO (2012) Spectrofluorimetric Assessment of
Hydrochlorothiazide Using Optical Sensor Nano-Composite
Terbium Ion Doped in Sol-Gel Matrix. J.Fluoresc 22: 827-
834.
- Bagher G M, Mehdi K (2013) Simultaneous Voltammetric
Determination of Captopril and Hydrochlorothiazide on a
Graphene/Ferrocene Composite Carbon Paste Electrode.
Electroanal 25: 1263-1270.
- Ziyatdinova GK, Budnikov GK Pogorel'tsevVI (2006)
Determination of captopril in pharmaceutical forms by
stripping voltammetry. J. Anal. Chem 61: 798-800.
- Absalan G, Akhond M, Karimi R, (2018) Simultaneous
determination of captopril and hydrochlorothiazide by using a
carbon ionic liquid electrode modified with copper hydroxide
nanoparticles. Microchim. Acta 185: 97.
- Karimi-MalehH, AhanjanK, Mehdi T, Ghaemyc M (2016) A
novel voltammetric sensor employing zinc oxide nanoparticles
and a new ferrocene-derivative modified carbon paste
electrode for determination of captopril in drug samples.
Anal.Meth 8: 1780-1788.
- Karimi-Maleh H, Ensafi AA Allafchian AR (2010) Fast
and sensitive determination of captopril by voltammetric
method using ferrocenedicarboxylic acid modified carbon
paste electrode. J Solid State Electrochem 14: 9.
- EnsafiAA,Karimi-MalehH,Mallakpour S,Rezaei B (2011)
Highly sensitive voltammetric sensor based on catecholderivative-multiwall carbon nanotubes for the catalytic
determination of captopril in patient human urine samples.
Coll. Surf. B: Biointerfaces 87: 480-488.
- Karimi-Maleh, H.Ganjali, M R,Norouzi, P,Bananezhad, A
(2017) Amplified nanostructure electrochemical sensor for
simultaneous determination of captopril, acetaminophen,
tyrosine and hydrochlorothiazide. Mater. Sci.Enginer 73:
472-477.
- Abdel Razak O (2004) Electrochemical study of
hydrochlorothiazide and its determination in urine and tablets.
J. Pharm. Biomed. Anal 34: 433-440.
- PARHAM H, ZARGAR B (2005) Square-wave voltammetric
(SWV) determination of Captopril in reconstituted serum and
pharmaceutical formulations. Talanta 65: 776-780.
- GorskaaA,Paczosa-BatoraB, SzlosarczykbM, Piecha (2022)
Highly sensitive voltammetric determination of captopril on
renewable amalgam film electrode. Talanta 273: 122937.
- Siangproh W, Ngamukot P, Chailapakul O (2003)
Electrochemical determination of captopril at boron-doped
diamond thin film electrode applied to a flow injection system.
Sensors and Actuators B: Chemical 91: 60-66.
- Purushothama H T, Arthoba N Y (2017) Electrochemical
study of hydrochlorothiazide on electrochemically pre-treated
pencil graphite electrode as a sensor. Sensing and Bio-Sensing
Res 16: 12-18.
- 127.Habibi D, Faraji A R, Gil A (2013) A highly sensitive
supported manganese-based voltammetric sensor for the
electrocatalytic determination of captopril. Sensors and
Actuators B: Chemical 182: 80-86.
- Shahbakhsh M, Noroozifar M (2019) 2D-Single-crystal
hexagonal gold nanosheets for ultra-trace voltammetric
determination of captopril Microchim. Acta 186: 195.
- Machini WBS, David-Parra DN, Teixeira MFS (2015)
Electrochemical investigation of the voltammetric
determination of hydrochlorothiazide using a nickel hydroxide
modified nickel electrode. Materials Science and Engineering
57: 344-348.
- Karimi-Maleh H, Moazampour M, Gupta VK,Sanati AL
(2014)Electrocatalytic determination of captopril in real
samples using NiO nanoparticle modified (9,10-dihydro-9,10-
ethanoanthracene-11,12-dicarboximido)-4-ethylbenzene1,2-diol carbon paste electrode. Sensors and Actuators B:
Chemical 199: 47-53.
- Tajik S, AflatoonianMR, Beitollahi H, Shoaie IS, Dourandish
Z, et al. (2020)Electrocatalytic oxidation and selective
voltammetric detection of methyldopa in the presence of
hydrochlorothiazide in real samples. Microchem J 158:
105182.
- Akbari CS, Krimi H, Keyvanfard M, Alizad K (2016)
Voltammmetric Determination of Captopril Using Multiwall
Carbon Nanotubes Paste Electrode in the Presence of
Isoproterenol as a Mediator. Iran. J. Pharm. Res 15: 107-117.
- Niazi A, Pourghobadi Z, Nematollahi D, Beiginejad H (2014)
Electrochemical Oxidation and Voltammetric Determination
of Captopril Using 4. Biphenol as a Homogeneous Mediator.
J. Electrochem Soc 161: H284-H289.
- Mazloum-Ardakani M,Sabaghian F,Khoshroo A, Abolhasani
M,Naeimi H (2015) Electrochemical determination of
captopril in the presence of acetaminophen, tryptophan, folic
acid, andl-cysteine at the surface of modified carbon nanotube
paste electrode. Ionics 21: 239-250.
- Purushothama HT, Arthoba NY (2019) Pencil graphite
electrode based electrochemical system for the investigation
of antihypertensive drug hydrochlorothiazide: An
electrochemical study Chem. Phys. Lett 734: 136718.
136.Salamanca-Neto CAR, EiseleAPP, RestaVG, ScreminJ,
Sartori ER (2016)Differential pulse voltammetric method
for the individual and simultaneous determination of
antihypertensive drug metoprolol and its association with
hydrochlorothiazide in pharmaceutical dosage forms Sensors
and Actuators B: Chemical 230: S0925400516302209.
View PDF

