Author(s): <p>Tan Ying An, Kavitha Nagandla* and Krishna Kumar A/L Hari Krishnan</p>
Cesarean scar pregnancy is a rare type of ectopic pregnancy but potentially life threatening. The incidence of this ectopic pregnancy continues to rise due to global increase in cesarean sections rates as well as the diagnosis with wide use of transvaginal ultrasound. Delay or wrong diagnosis may result to uterine rupture and life-threatening bleeding with potential maternal morbidity and mortality. Endovaginal ultrasound has a reported sensitivity of 85-90 % for detection. In difficult cases, magnetic resonance imaging is often useful as second line imaging. Treatment may be ranging from conservative to medical therapy or surgery. We present a series of three illustrative cases successfully managed with medical management and sequential treatmentof medical and surgical management. We discuss diagnostic challenges and review of literature on updates on management strategies.
Caesarean scar pregnancy is the implantation of the gestational sac into the myometrial defect occurring at the site of the previous uterine incision. This is a potentially life-threatening type of ectopic pregnancies with incidence of approximately1 in 2000 pregnancies [1]. The incidence of CSP is increasing due to increased number of cesarean deliveries and the widespread use of Ultrasound (US) in early pregnancy [2]. There are two types of CSP - Type I (endogenic type): A gestational sac implanted in the scar that grows into the uterine cavity and type II (exogenic type) is implanted in the myometrium and mainly grows towards the abdominal cavity. Type II is a high-risk clinical situation with more complex presentations associated with uterine rupture and bleeding [3]. Early diagnosis is paramount in successful conservative management, optimizing complication and fertility preservation [4]. The diagnostic features are as identified by transvaginal sonography with the presence of a gestational sac at the site of the previous cesarean scar and an empty uterine cavity [5,6]. Few other possible ultrasonographic features include evidence of trophoblast invasion between the bladder and the anterior uterine wall appearing as bulging sac, deficiency of myometrium the between gestational sac and bladder with discontinuity of the anterior uterine wall in the sagittal plane and doppler findings of perfusion of the peritrophoblastic vasculature [7,8].
Various treatment options have been proposed. however, the optimal treatment is not unclear. Medical management includes methotrexate, which may be administered by local injection into the gestational sac under ultrasound guidance or systemically by intramuscular injection. Surgical treatment consists of either evacuation of the pregnancy (using suction or hysteroscopic resection) and excision of the pregnancy as a laparotomy, laparoscopic or transvaginal procedure. There is evidence that management options should ideally be tailored according to the patients clinical and sonographic presentation, beta human chorionic gonadotropin (hCG) levels, and the surgeon’s experience [9-11].
The aim of this study is to present case series of cesarean scar pregnancy at hospital Tunaku Jaffar, Seremban, a tertiary hospital since 2020 until 2022, with different types (endogenous and exogenous) and their management strategies. Furthermore, the paper describes the evolution of diagnosis, treatment modalities, and outcomes as reported in literature.
This is a retrospective case series of 3 patients with diagnosed caesarean scar pregnancy between 2021-22. The diagnosis was confirmed by transvaginal ultrasound examination. Clinical data and findings are presented below,
A 34-year-old woman, G3P2 with 2 previous caesarean section presented with vaginal bleeding and lower abdominal pain to our center. She was diagnosed to have missed miscarriage and underwent evacuation of uterus. Only minimal tissue was evacuated during operation. Blood loss was minimal and the patient was discharged well on the next day. She presented to us after one month with the complaint of continuous vaginal bleeding following discharge from the hospital. Ultrasound scan noted a mass with mixed echogenicity 7x4cm with increased Doppler uptake at the lower part of the uterus with myometrium thickness <0.5cm. MRI was proceeded to confirm the diagnosis. Findings are suggestive of a well-encapsulated heterogeneous mass is seen arising from the endometrial cavity in the anterior part lower uterine segment 6.4x7.7x6.7cm. There is fluid component within the mass with pancake shape heterogenous soft tissue attached to the anterior wall. No fetal part seen. The adjacent myometrial layer is significantly thinned out, suspicious of infiltration. Adjacent serosa layer is intact. Initial serum beta hcg 1656 IU/L. She was treated with one dose of intramuscular Methotrexate 1mg/kg. Subsequently she was on follow up with weekly ultrasound scan and beta hcg level. It took 5 weeks until the beta hcg archived to the normal level.

Figure 1: Ultrasound Scan Noted a Mass with Mixed Echogenicity 7x4cm at the Lower Part of the Uterus
A 38 years old woman G3P2 with 1 previous LSCS presented with per vaginal spotting for 1 week. Ultrasound scan noted retroverted uterus with possible gestational sac implanted at the anterior lower part of the uterus, with increased doppler uptake (Figure 2). MRI shows heterogenous ill- defined lesion 4.8x4.8x5.1cm at the anterior lower uterine region. The lesion infiltrate more than ½ half of the myometrium with intact serosal layer. Initial serum β-hCG 817 IU/L. She received first dose of intramuscular Methotrexate and subsequently follow up weekly with ultrasound scan and serum β-hcg. As β-HCG was static after one month, a 2nd dose of methotrexate was given. β-Hcg normalized 1 month after the 2nd dose of Methotrexate.

Figure 2: Ultrasound Scan Noted Retroverted Uterus with Possible Gestational Sac Implanted at the Anterior Lower Part of the Uterus
A 35 years old women with two previous LSCS presented to us with the complaint of per vaginal bleeding for 1 week. Ultrasound scan noted a gestational sac 1.4cm with small fetal pole and yolk sac at the caesarean scar site. (Figure 3) The myometrium thickness at that area is <0.5cm. Initial β-hCG 17906. Local injection of methotrexate into the gestational sac was unsuccessful. however, procedure was not successful. Patient was counselled for surgery in view of β-hCG. Intra-op noted bulging 3x2cm at left angle of the previous caesarean scar (Figure 4). Only serosa without normal myometrium covering the gestational sac. Resection was done and patient was discharge well after 2 days.

Figure 3: Ultrasound Scan Noted a Gestational Sac 1.4cm with Small Fetal Pole and Yolk Sac at the Caesarean Scar Site
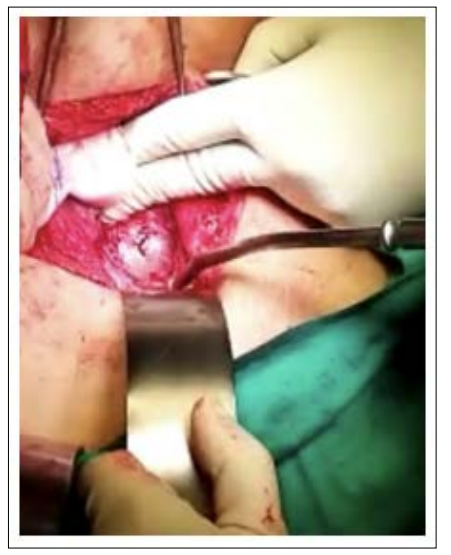
Figure 4: Intra-op Noted Bulging 3x2cm at Left Angle of the Previous Caesarean Scar
Cesarean Scar Pregnancy (CSP) is the implantation of a gestational sac in the myometrium of a previous cesarean scar [2]. There is still uncertainty about the exact pathophysiology of CSP, however it is to believe that defect at the caesarean scar as a result of inappropriate repair or healing can predispose the women to CSP [5]. We will discuss management of CSP under aspects of initial diagnostic evaluation, management and further monitoring and prediction of future recurrences.
The symptoms of CSP are unspecific, and one-third of cases are asymptomatic [12]. In our series, all our 3 patients were only presented with per vaginal spotting. Hence the recommendation to perform a routine early first-trimester sonogram in patients with previous cesarean section for an early diagnosis. However, studies have shown that there are often some missed diagnoses of CSP by ultrasound diagnosis, and hence the need for diagnostic tools with more efficacy [13]. There is growing interest in the role of MRI in recent years in the diagnosis of CSP. 3.0T MRI is especially considered a safe gestational imaging to have a high clinical effectiveness in diagnosis. Hoffmann et al. compared two diagnostic methods to diagnose 25 CSP patients and highlighted a significant difference between the diagnostic results of 3.0T MRI and ultrasound with 3.0T MRI could clearly image the uterine scar in 44% of the patients and had a high accuracy in measuring the wall thickness of the lower uterus [14]. These results were reproduced by recent study by Guo, Shuning et al supporting the higher clinical value of 3.0T MRI [16]. In our case series, all cases were subsequently confirmed with MRI. Nevertheless considering the availability of resources, transvaginal ultrasound will be the primary imaging modality to diagnose CSP with sensitivity of 86,4% [13].
The choice of treatment should take into consideration β-hCG level, size of the gestational sac, availability of the expert and women’s wish. The options with evidence of effectives thus far include systemic or local Methotrexate (MTX) administration, dilation and evacuation (D&E), uterine artery embolization, hysteroscopy, and laparoscopy removal of CSP [15]. The expectant management has been reported in endogenous or type 1 CSP because it grows toward the uterine cavity although there is still a potential risk of uterine rupture, life-threatening massive bleeding, and hysterectomy which must be communicated [16]. Therefore, expectant management may not be a good fertility-preserving option. Local or systemic administration of Methotrexate is one of the most popular treatments for CSP because it is noninvasive and lesser risks. In a study by Bodur et al. primary systemic MTX administration was effective for a cesarean scar ectopic pregnancy especially prior to 8 weeks of gestational age, and with β-hCG concentration of ≤ 12,000 mIU/ml, and absent embryonic cardiac activity [17]. A clinical trial study shows that a single dose of systemic methotrexate is equally successful as the local administration of methotrexate (67.3% vs 69.2%, respectively). However, the decline of the serum β-hCG level and pregnancy disappearance were faster in the systemic group. In the present study, two patients were successfully treated with a primary systemic MTX administration. In a study by Levin et al., the majority were successfully treated with systemic injection of MTX, some patients required with a combination of systemic and local as intra-sac administration [18]. One of our patients had unsuccessful local therapy and subsequently required surgical intervention. It is postulated that presence of fibrous scar rather than normally vascularized myometrium around the pregnancy minimises the systemic absorption of local MTX and delay complete resorption of the pregnancy. [19].
The surgical approach involves cervical dilatation and curettage in type 1 or endogenous CSP with myometrial thickness at least 2mm . However, there is a risk of bleeding and failure of complete gestational sac removal [20]. Surgical resection abdominally or laparoscopically is performed for exogenous type of CSP thin myometrium. Our case 3 was successfully managed with surgical resection. There is evidence to support the effectiveness of sequential treatment of curettage following medical treatment has a high rate of success and no significant effects on the intraoperative bleeding, shorter time of therapy and a more favorable outcome [21]. Another study reported success with methotrexate administration followed by suction curettage with Foley tamponade [22].
Uterine Artery Embolization (UAE) has been proposed in patients with haemorrhage following medical treatment or conservative surgery [23]. There is a role of UAE pre-treatment which has proven to reduce blood loss and duration of hospitalization. However, in women desirous of future fertility, should be counseled regarding the risks of pregnancy such as preterm labor, malpresentation, miscarriage, and postpartum hemorrhage. Hence UAE is not considered a first-line option for patients who desire future fertility [24].
Serial hCG and TVUS color Doppler are the choice of investigations in monitoring the treatment outcomes. There appears to be a good correlation between the hCG values and persistence of the trophoblastic flow at the site of CSP implantation [25]. In all our cases, doppler examination demonstrating persistent functional trophoblast was main strategy of surveillance of the response to treatment.
There is paucity of data on risk of recurrence of the condition in future pregnancies and if the interval between previous caesarean delivery and recurrence of CSP [23]. However, there are reassuring reports on overall good fertility outcomes following CSP and need for early surveillance in future pregnancy [24]. We hope there will be development of risk scoring system and CSP registry in future that will guide management options. This case series is discussed in the interest of contributing to existing case series/ reports of CSP [26].
With increasing caesarean rates and recent covid policy of elective caesarean section, it is anticipated that clinicians will encounter CSP from time to time. An accurate and early diagnosis of CSP can result in successful termination of pregnancy and preventable complications. Despite the numerous case series with experience shared by authors, there is yet a risk scoring system in place to guide management options. We hope our case series contributes our experience to building the evidence for the management of this complex clinical presentation.
Funding: This article has not been funded by any public/private external entity or any commercial organization.
Informed Consent Statement: The authors have obtained the patients’ signed consent to publication. This work has not been published before and it is not under consideration for publication anywhere else.
Guarantor: Tan Ying An
Conflicts of Interest: In this study, there is no conflicts of interest.
