Author(s): Feyza Yasar Dasgin
Brain metastases of solid tumors in childhood are rare events. Clear cell sarcomas of the kidney, which are among the causes of brain metastasis observed in this age group, constitute only 2-3% of kidney tumors. Even though clear cell sarcoma of the kidney and their metastases are rarely seen, they have a poor prognosis. In this case report and a brief review of the relevant literature, we aimed to summarize the diagnosis, follow-up, and treatment processes of pediatric cases with brain metastasis due to clear cell sarcoma. Changing of clear cell sarcoma treatment planning in recent years and the possibility of difference in relapsing pattern because of this change are also mentioned in this review.
Renal tumors are the 5th most seen cancer in children under 15 years. The clear sarcomas of the kidney are 2-3% of these renal tumors [1]. Although clear sarcomas of the kidney are rarely seen tumors, they are the second most common pediatric renal tumors after Wilms tumors [2]. Once considered a Wilms tumor variant, the kidney’s clear sarcoma was accepted as a separate entity after being described in 1970 by Kidd [3]. The clear sarcoma of the kidney differs from Wilms tumor with male gender predominance; distant metastases are rarely seen in the diagnosis, brain and bone metastases are fewer seen and worse prognosis than Wilms tumor [4,5].
Brain metastases frequently exist in adult cancer patients, but this is scarce in the pediatric patient population. According to publishing clinical data, as brain metastases in adult cancer patients are seen %20-40 frequency, this frequency is 1-10 % in pediatric cancer patients [6-9]. Brain and bone metastases are rarely seen in pediatric cancer patients; to the contrary, they are seen frequently in the kidney’s clear cell sarcomas. At the same time, brain metastases of clear cell sarcoma cases are few in the literature. A total of 8609 patients with Wilms tumor were enrolled on the National Wilms Tumor Study (NWTS) clinical trials between 1969 and 2002. In the following of these patients, intracranial metastases happened only in 47 patients, and the brain metastases rate of incidence is around 0,05%, according to this study [10]. However, brain metastases are more seen than bone metastases in clear cell sarcoma of the kidney according to NWTSG and SIOP (International Society of Pediatric Oncology) recent reports [11,12].
We aimed to explain in our study that one child patient had brain metastases of clear cell sarcoma of the kidney, and because of that, we applied radiotherapy to him and his treatment results. Also, case reports in the literature that are treatment options of brain metastases of clear cell sarcoma are evaluated are compiled in our study
A 9-month-old male patient with no known malignancy and immunodeficiency and no known hereditary disease in his family history was admitted to the hospital with the complaint of abdominal enlargement and swelling for two weeks. On physical examination of the patient, a right-sided palpable mass was detected in the abdomen. A mass of 115x100x130 mm originating from the right kidney and compressing the inferior vena cava was detected in the abdominal ultrasonography. Computed tomography (CT) taken for abdominal imaging revealed a 12x12x13 cm solid mass originating from the right kidney, extending to the left of the bony pelvis and midline, and containing necrotic hemorrhagic areas. While the pathology result of the patient who underwent right nephrectomy was clear cell carcinoma, it was reported that the mass was infiltrated into the renal capsule, renal pelvis, and renal sinus with a positive surgical margin but no apparent vascular invasion.
After surgery, one dose of vincristine was given at a dose of 0.05 mg/kg to the patient who had no distant metastases. A total of 1050 cGy conformal radiotherapy (RT) was applied to the whole abdomen with 150 cGy x 7 fractions.
After radiotherapy (NWTSG)-5 Regime I (vincristine, doxorubicin, etoposide, cyclophosphamide) 24-week protocol was applied. According to the abdominal and thorax tomography images taken at the end of the protocol, the patient was evaluated in remission and was followed up.
At the end of a one-year follow-up period, the patient presented with complaints of weakness, drowsiness, disturbance in eating, gait disturbance, deviation in the left eye that progressed within one week. The patient’s emergency brain CT showed a lesion compatible with approximately 55x38x48 mm metastasis on the left in the posterior fossa, accompanying tri-ventricular acute hydrocephalus and 8-9 mm herniation of the cerebellar tonsils from the foramen magnum to the inferior (Figure 1a-1b).
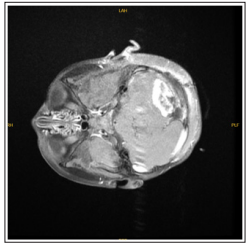
Figure 1a: An axial section taken from the T1 weighted-contrast enhanced sequence in MR
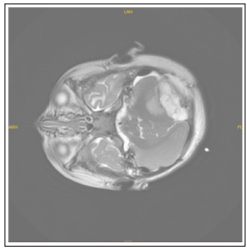
Figure 1b: An axial section taken from T2 weighted MRI
Figure 1a-b: Preoperative MR images showing brain metastasis of a patient diagnosed with clear cell sarcoma of the kidney. In the posterior fossa, there is a lesion compatible with a metastasis approximately 55x38x48 mm in size on the left.
The patient was taken into emergency surgery for mass excision due to these findings and increased intracranial pressure. Excision material was reported as posterior fossa brain parenchyma containing clear cell sarcoma metastasis.
The patient was referred to our clinic after the post-op Brain MRI (magnetic resonance imaging) revealed residue in the left cerebellar hemisphere’s operation cavity and another smaller nodular metastasis at the left cerebellar tonsil level (figure 2). Whole-brain radiotherapy was planned for the patient on the 30th postoperative day at a dose of 1.8 Gy x 12 fractions. A boost dose was not planned due to the severe edema effect and the patient’s young age. After CT simulation under anesthesia for the patient’s immobilization, the treatments were also applied under anesthesia.
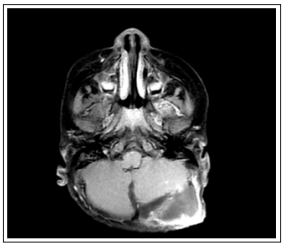
Figure 2: BAn axial section of the T1-weighted-contrast-enhanced sequence taken from the pre-radiotherapy MR after surgery with brain metastases with a diagnosis of clear cell sarcoma of the kidney. In the left cerebellar hemisphere, there is residual metastasis in the operation cavity and another smaller nodular metastasis at the level of the left cerebellar tonsil
On the 16th day after completing the radiotherapy, ICE (ifosfamide, carboplatin, etoposide) chemotherapy protocol started. After chemotherapy, the patient, who was under follow-up due to typhlitis and febrile neutropenia after chemotherapy, had a control brain MRI taken at the 2nd month after RT, without contrast. Although imaging was not optimal, it was reported that the nodular appearance observed in the left cerebellar tonsillar neighborhood disappeared (figure 3).
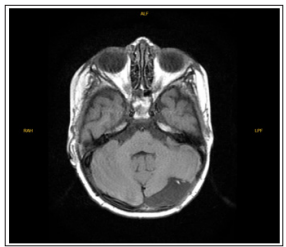
Figure 3: It is an axial section taken from the T1-weighted-contrast enhanced sequence from the MR of the patient diagnosed with clear cell sarcoma of the kidney, taken 2 months after radiotherapy. It is observed that the nodular appearance observed in the left cerebellar tonsillar neighborhood seen in the previous MR has disappeared.
After RT, the patient received the 3rd cycle of chemotherapy without any problem. It was decided that the patient, whose general condition was good and whose systemic treatment was continuing, would be followed up with a brain MRI every two months.
Clear cell sarcoma of the kidney accounts for only 2-3% of kidney tumors. Clear cell renal sarcoma and metastases of these tumir are rare. Brain metastasis is a rare event in solid childhood tumors. The life expectancy of brain metastasis of childhood tumor is poor. Combination of surgical resection, chemotherapy and radiotherapy may prolong life expectancy in these patients. Among patients with solitary brain metastases, those who undergo total surgical resection have a longer life expectancy; and chemotherapy agents also increase the treatment success of patients. In addition, RT reduces the neurological progression of patients and improves survival. There is limited data on clear cell sarcoma of kidney in the literature. There is no standard dose of radiotherapy recommended for clear cell sarcoma metastases of the kidney. Recent studies show that the brain metastases of clear cell sarcoma of kidney have increased. Brain metastases due to childhood renal sarcomas can be observed in more cases in the future due to changes in treatment regimens. Experiences and treatment options for clear cell sarcomas of the kidney in childhood should be shared more and a guide should be created about this rare disease and this metastases.
Central nervous system (CNS) involvement in childhood cancers is mainly observed in leukemias. Brain metastasis development due to solid tumors is very rare in this age group. Several different reasons suggested for the less frequent observation of this entity, which is common in adults in solid childhood tumors. The first is that disease histopathology and behavior differ between these two populations. Among the reasons suggested is the immaturity of the blood-brain barrier in children and the hypothesis that systemic agents may have more CNS transmission in this age group [9]
Clear cell sarcomas of the kidney, a significant cause of brain metastases in children, were previously considered a variant of Wilms’ tumor; It was defined as a separate entity in 1970 by Kidd et al. And named as clear cell sarcoma by Beckwith and Palmer [3,13].
The average age of onset for clear cell sarcomas of the kidney is 36 months, and the most common clinical manifestations are abdominal mass and hematuria. The most commonplace of metastases are in lymph nodes (59%), followed by bone (13%), lung (10%), and liver (9%). Relapses are seen in approximately 20-40% of the diagnosed cases.
The mean time to recurrence after the end of treatment is defined as 24 months [14]. In this case, the diagnosis was made when they presented with an abdominal mass at the age of 9 months, and the disease was treated and followed up according to the current treatment and follow-up protocols. In this case, the relapse in the brain is 12 months after diagnosis.
The most effective treatment method for clear cell sarcomas of the kidney is the combination of surgery, chemotherapy, and radiotherapy. Patients’ prognosis improved dramatically with the addition of doxorubicin (D) to vincristine (V) actinomycin (A) therapy [15]. In the NWTS-3 study, adding low-dose cyclophosphamide to this combination did not improve patient outcomes. In NWTS-4, the results of 6 months of VDA treatment and 15 months of VDA treatment were compared; It has been reported that 8-year relapse-free survival has increased (87.8% vs. 60.6%, p = 0.08), but no difference was observed in overall survival (87.5% vs. 85.9%) [16]. In NWTS-5, patients were treated with cyclophosphamide/etoposide in addition to the VDA regimen for six months. In preliminary studies, event-free survival and overall survival were 79% and 89%, respectively [15-18].
In the last COG (Children’s Oncology Group) study, the same regimen as in the NWTS-5 was applied for stage I-III patients; the only stage I patients was not given abdominal RT. Stage IV patients, as in diffuse anaplastic Wilms tumor, were treated with vincristine, doxorubicin, and cyclophosphamide, followed by cyclophosphamide, carboplatin, and etoposide [1].
Studies have reported that pediatric patients with clear cell renal sarcoma have a high risk of late recurrence. Therefore, longterm follow-up of patients is recommended [4]. Renal clear cell sarcomas can be fatal due to local recurrence, late recurrence, and systemic metastases such as brain and bone.
Brain metastases are extremely rare in solid childhood tumors, and parallel to this, there is limited information in the literature on this subject. The most common symptoms and signs in these cases are; nausea, headache, hemiparesis, limb weakness, and balance loss [19,20]. Due to the rarity of these cases, there is no clear consensus on their treatment. Factors such as the patient’s age, the severity of the symptoms due to metastasis, the type of primary disease, the chance of surgery, and the general condition of the patient determine the treatment approach. Based on this case, the Pubmed database was scanned. The case reports we obtained through the searches made in the literature between 1985-2020 in English as ‘clear cell sarcoma of kidney with brain metastases’ and ‘clear cell sarcoma of kidney’ are summarized in the Table 1. Age of primary disease, age of diagnosis of brain metastasis, and treatment given are explained in this Table 1.
Table 1: Case reports of brain metastasis due to clear cell sarcoma of the childhood kidney| Patient | Age of onset | Brain metastases diagnosis age | Treatment | Treatment |
|---|---|---|---|---|
| 1 | 3 y | 5,5 y | Chemotherapy - total excision -- Chemotherapy |
[13] |
| 2 | 10 mo | 20 mo | 20 Gy x 2 frx radiotherapy - total excision - 28,6 Gy x 12 frk radiotherapy | [14] |
| 3 | 4y | 4 y 8 mo | Total excision-15 Gy total brain radiotherapy -- Chemotherapy | [21] |
| 4 | 5,5 y | 6 y | [22] | |
| 5 | 19 mo | 2 y | [22] | |
| 6 | 12 mo | 20 mo | Total excision- radiotherapyChemotherapy | [23] |
| 7 | 2 y | 35 mo | Total excision - Chemotherapy | [24] |
| 8 | 11y | 14 y | [25] | |
| 9 | 9 mo | 20 mo | Radiotherapy(total 60 Gy) - Chemothreapy | [26] |
The average life expectancy of patients with untreated brain metastases is around one month [27-29]. The combination of surgical resection, chemotherapy, and radiotherapy (RT) constitutes most of the treatments for brain metastasis in children. According to Paulino et al., RT decreases the neurological progression of patients and increases survival [25].
According to recent studies, treatment success increases depending on chemotherapeutic agents, including ifosfamide and etoposide. Among patients with solitary brain metastases, those with more prolonged survival often have total surgical resection [14].
There is no standard dose of radiotherapy recommended for clear cell sarcoma metastases of the kidney. In patients with brain metastases, treatment can be given to the whole brain at doses of 21.6 Gy or 30.6 Gy. When a dose of 30.6 Gy is given to the whole brain, an additional boost dose is not required. In patients younger than three years of age, in the presence of ≤ 3 brain metastases (tumor or tumor bed 0.5cm margin), after the whole brain received 21.6 Gy of radiotherapy, a total of 10.8 Gy boost dose can be given in 6 fractions [30]. In this case, surgical resection was performed first since it was the only lesion for brain metastasis.
Subsequently, a total of 21.6 Gy in 12 fractions was given to the whole brain. The boost dose was not administered in this case due to the high effect of edema, the patient’s younger age, and the increased risk of complications due to anesthesia.
Although clear cell sarcomas of the kidney are tumors that cause bone or lung metastases, according to the literature, recent studies show that the brain metastases of these tumors have increased. In NWTS-5, the first relapse in 12 of 23 metastatic patients was in the form of brain metastasis. This differs with NWTS-4, where the most common recurrence areas are bones and lungs [31]. However, in another study, relapse occurred in 37 of 237 clear cell renal sarcoma patients who received treatment according to SIOP and AIEOP protocol. The most common sites of relapse are the brain, lungs, and bone [2]. It is thought that the likely cause of this change in the recurrence pattern may be the changing chemotherapy regimen. Cyclophosphamide, which is used as a chemotherapeutic agent, can pass to the central nervous system more than the other three agents in NWTS-4, thus creating a better chemotherapy response than other agents. Another reason for the change in the recurrence pattern may be that the chemotherapeutics in this protocol kill residual cells in other recurrence areas, such as bone and lungs. In contrast, previous chemotherapeutics may not achieve adequate control in the brain [2,31].
In conclusion, brain metastases due to childhood renal sarcomas can be observed in more cases in the future due to changes in treatment regimens. On the other hand, pediatric cases are observed. As a result, the possibility of brain metastasis due to childhood kidney tumors increases compared to recent studies. In this age group, experiences and opinions about brain metastases’ treatment due to solid tumors should be shared more, and standard treatment schemes with consensus should be established to treat this rare clinical condition
