Author(s): Abu OD*, Iyare HE and Ogboi KU
The aim of this study was to investigate the antioxidant property of total saponins and tannins of Dialium guineense stem bark in rats hearts exposed to carbon tetrachloride (CCl4). A total of twenty-five (25) adult male Wistar rats, which weighed between 170 and 190 g (mean weight = 180 ± 10 g) were randomly assigned to five groups (5 rats per group): normal control, CCl4 control, silymarin, total saponins and total tannins groups. With the exception of normal control group, the rats were exposed to CCl4 (single oral dose of 1.0 mL/kg body weight, bwt). Rats in the silymarin group were administered 100 mg silymarin/kg bwt (standard cardioprotective drug), while those in the two treatment groups received 150 mg/kg bwt of total saponins or tannins orally for a period of 28 days. Activities of antioxidant enzymes such as catalase, superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) were evaluated in heart homogenate. The results showed that there were no significant differences in the concentrations of cardiac total protein (TP) among the groups (p > 0.05). The activities of the antioxidant enzymes and level of reduced glutathione (GSH) were significantly lower in CCl4 control group than in normal control group, but they were increased by total saponins/tannins treatment (p < 0.05). However, the level of cardiac malondialdehyde (MDA) elevated by CCl4 intoxication was markedly reduced after treatment (p < 0.05). These results indicate that total saponins and tannins of D. guineense stem bark may enhance antioxidant defense in rats hearts exposed to CCl4.
The heart is a muscular organ which pumps blood through the blood vessels of the circulatory system [1]. Blood provides the animal?s body with oxygen and nutrients as well as assist in the removal of metabolic wastes. In humans, the heart is located between the lungs in the middle compartment of the chest [2- 4]. The heart is effectively a syncytium, a meshwork of cardiac muscle cells interconnected by contiguous cytoplasmic bridges [5-7]. Heart disease is caused by a multitude of chemicals, drugs, herbal and dietary supplements. A good example of such toxic agent is CCl4 .
Myocardial infarction (MI), characterized by the hypoxic state of myocardial tissue sequel to uneven perfusion of coronary blood supply compared to demand, is a leading cause of cardiovascular morbidity and mortality [8]. It causes myocardial ischemic injury and damage to the cardiomyocytes. The repair of damaged hearts in patients is challenging due to the limited ability of the cardiomyocytes to regenerate post-mitotically. Prolonged evident ischemia in chronic MI eventually leads to permanent myocardial cell injury or death [9]. Studies suggest that the accumulation of reactive oxygen species (ROS) during ischemic damage plays a pivotal role in the pathogenesis of heart disease with the attendant deleterious consequence, evidenced by the elevation of cardiac troponin-I (cTn-I), the up-regulation of the pro-inflammatory cytokines interleukine-1 (IL-1b) and IL-6, and tissue necrotic factor α (TNF-α) [9-11].
The use of plant-derived compounds with antioxidant properties is of great interest to Scientists due to the overwhelming evidence implicating oxidative stress in the pathogenesis of heart diseases [12]. Similarly, the limitations (such as a high level of toxicity and low aqueous solubility) observed following the use of synthetic antioxidants in preventing cardiovascular diseases have shifted the attention of researchers to the use of naturally derived antioxidants, owing to their high safety margin, cultural acceptability, and promised effectiveness [13,14]. The aim of this study was to investigate the antioxidant property of total saponins and tannins of D. guineense stem bark in rats hearts exposed to CCl4 .
All chemicals and reagents used in this study were of analytical grade and they were products of Sigma-Aldrich Ltd. (USA).
The stem barks of D. guineense were obtained from Auchi Area of Edo State, Nigeria and authenticated at the herbarium of the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria (No. UBH33D).
The stem bark was washed and shade-dried at room temperature for a period of two weeks and crushed into small pieces using clean mortar and pestle. Total saponins and tannins were isolated from the plant stem bark using standard methods [15,16].
Adult male Wistar rats (n = 25) weighing 170 - 190 g (mean weight = 180 ± 10 g) were obtained from the Department of Anatomy, University of Benin, Benin City, Nigeria. The rats were housed in metal cages under standard laboratory conditions: temperature of 25 °C, 55 - 65 % humidity and 12-h light/12-h dark cycle. They were allowed free access to rat feed (pelletized growers mash) and clean drinking water. Prior to commencement of the study, the rats were acclimatized to the laboratory environment for one week. The study protocol was approved by the University of Benin Faculty of Life Sciences Ethical Committee on Animal Use.
The rats were randomly assigned to five groups (5 rats per group): normal control, CCl4 control, silymarin, total saponins and total tannins groups. With the exception of normal control, the rats were exposed to CCl4 (single oral dose of 1.0 mL/ kg bwt). Rats in the silymarin group were administered 100 mg silymarin/kg bwt, while those in the two treatment groups received 150 mg/kg bwt of total saponins or tannins orally for 28 days.
At the end of the treatment period, the rats were euthanized and their heart excised, and used to prepare 20 % tissue homogenate. The homogenate was centrifuged at 2000 rpm for 10 min to obtain supernatant which was used for biochemical analysis.
The activities of catalase, SOD and GPx were determined [17- 19]. Levels of total protein, MDA and GSH were also measured [20-22]. The activity of GR was determined using a previously described method [23].
Data are expressed as mean ± SEM (n = 5). Statistical analysis was performed using GraphPad Prism Demo (6.07). Groups were compared using Duncan multiple range test. Statistical significance was assumed at p < 0.05.
There were no significant differences in relative organ weight among the groups (p > 0.05; Table 1).
| Group | Relative organ weight x 10 -2 |
|---|---|
| Normal Control | 3.34 ± 0.54 |
| CCl 4 Control | 3.02 ± 0.10 |
| Silymarin | 3.17 ± 0.16 |
| T. Saponins | 3.34 ± 0.22 |
| T. Tannins | 3.21 ± 0.27 |
Data are relative organ weights and are expressed as mean ± SEM (n = 5). Where T. Saponins and T. Tannins = total saponins and total tannins, respectively.
There were no significant differences in the concentrations of cardiac TP among the groups (p > 0.05). The activities of the antioxidant enzymes and level of GSH were significantly lower in CCl4 control group than in normal control group, but they were increased by total saponins/tannins treatment (p < 0.05). However, the level of cardiac MDA elevated by CCl4 intoxication was markedly reduced after treatment (p < 0.05). These results are shown in Figures 1 to 3.
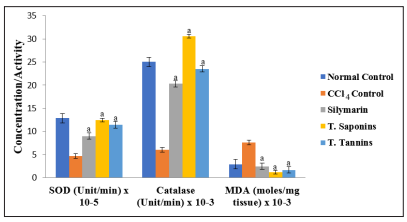
Figure 1: Comparison of the Effect of Total Saponins and Tannins of D. guineense Stem Bark on Markers of Oxidative Stress in Rat Heart. Data are oxidative stress markers, and are expressed as mean ± SEM. a p < 0.05, when compared with CCl4 control group.
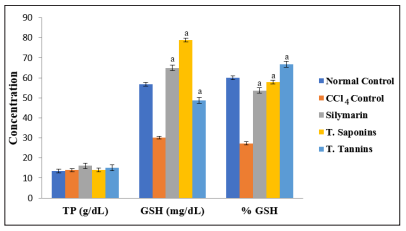
Figure 2: Effect of Total Saponins and Tannins of D. guineense Stem Bark on Some Oxidative Stress Parameters. Data are oxidative stress markers, and are expressed as mean ± SEM. a p < 0.05, when compared with CCl4 control group.
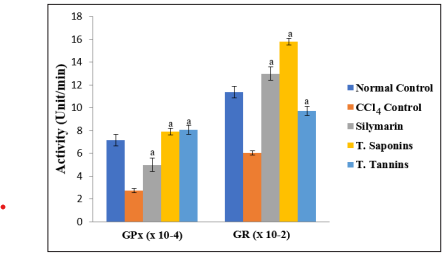
Figure 3: Comparison of the Effect of Total Saponins and Tannins of D. guineense Stem Bark on Rat Oxidative Status. Data are oxidative stress parameters, and are expressed as mean ± SEM. a p < 0.05, when compared with CCl4 control group.
Heart disease and related complications are the main causes of deaths throughout the world [24]. The use of herbal antioxidants is increasing as defensive agents against a number of cardiovascular abnormalities. Bioactive agents from natural sources have gained fundamental importance and wide acceptability in modern medicine, as they are known to reduce the risk of cardiac ailments by either scavenging free radicals or halting their formation [25]. Herbal medicines play considerable role in health care to a large proportion of the world?s population and have been regarded as component of cultural heritage of various tribes. Polyphenols exert cardioprotective effect via inhibition of the oxidation of low-density lipoprotein cholesterol (LDL-C) [26]. Most of the pharmacologically important drugs are derived from plants. Plant derivatives used as drugs play significant role in health-care systems around the globe. They are not only used for the management of disease conditions but also for maintenance of proper health [27]. The past decade revealed that plant-based herbal remedies are the emerging choice for the treatment of various ailments [28]. Most of the widely used modern medicines for the treatment of cardiovascular abnormalities have side effects and there is dire need to search for alternative therapies with fewer or no side effects.
Dialium guineense is a medicinal plant used in folklore medicine for the treatment of infections such as diarrhea, severe cough, bronchitis, wound, stomachaches, malaria, jaundice, ulcer and hemorrhoids [29]. The bark, leaves and fruits of D. guineense have medicinal properties and are used in Traditional Medicine to treat different diseases [30]. Phytochemical screening of the plant stem bark reveals the presence of bioactive compounds such as alkaloids, flavornoids, phenols, saponins, steroids and tannins [31]. These phytochemicals are believed to be responsible for its various biological and pharmacological effects [32,33]. Extracts of the plant have been shown to possess anti-inflammatory, antioxidant, antiplasmodial and hepatoprotective effects. This study investigated the antioxidant property of total saponins and tannins of Dialium guineense stem bark in rats hearts exposed to CCl4 . The results showed that there were no significant differences in the concentrations of cardiac total protein among the groups. The activities of the antioxidant enzymes and level of GSH were significantly lower in CCl4 control group than in normal control group, but they were increased by total saponins/ tannins treatment. However, the level of cardiac MDA elevated by CCl4 intoxication was significantly reduced after treatment.
The effects produced by total saponins and tannins of the medicinal plant were comparable with those of the standard cardioprotective drug, silymarin. The phytochemicals markedly ameliorated the cardiotoxicity induced by CCl4 .
In cardiomyocytes, the protective effect of medicinal plants/ herbal products may be exerted via mechanisms such as opening of KATP channel, increased secretion of atrial natriuretic peptide, and regulation of cardiac hypertrophy, oxidative stress, and apoptosis. In endothelial cells (ECs), the mechanisms may involve inhibition of inflammation, oxidative stress and apoptosis; endothelial nitric oxide synthase/nitric oxide (NOS/ NO) signaling pathway activation, angiogenesis induction, and endothelial permeability suppression. In vascular smooth muscle cells (VSMCs), the mechanisms include down-regulation of structural and contractile proteins activities/expression, modulation of extracellular matrix proteins/glycoproteins expression, regulation of calcium levels, alleviation of inflammation, attenuation of proliferation and migrations, as well as enhancement of mitochondrial function [34].
This study has demonstrated that total saponins and tannins of D. guineense stem bark could serve as natural therapeutic agents for clinical management of cardiovascular abnormalities induced by cardiotoxic agents.
