Author(s): Qinya Yi, Jilun Zhang and Di Ai*
Pseudomonas aeruginosa is a nosocomial pathogen that often develops multidrug resistance. Treating P. aeruginosa infection can be difficult, high cost with high mortality rate. Novel treatment is needed to handle this common clinically encountered issue. Recent research has suggested that Essential Oils (EOs) have the potential to act as antibiotics on P. aeruginosa by inhibiting the formation of biofilms and the functions of efflux pumps. Because of lower cost, EOs have huge potential for developing countries and underdeveloped area. Innovative therapeutic technologies, such as nano-encapsulation of EOs, have been utilized to enhance EOs’ effectiveness. This review summarizes the mechanisms of EOs in P. aeruginosa treatments, the current application of EOs, and future developments of EOs in and beyond clinical practice.
Antimicrobial Resistance (AMR) is a protective mechanism developed by bacterium, often using its inherent bacterial structures and components to eliminate the effectiveness of previously potent antibiotics. Pseudomonas aeruginosa (P. aeruginosa), an opportunistic pathogen known for its strong ability to adapt to different environments and gain complex pathogenicity (Figure 1), displays a variety of resistance mechanisms, acquiring high levels of intrinsic antibiotic resistance. P. aeruginosa develops AMR through its low permeability of the outer membrane, efflux systems that pump antibiotics out, and production of antibiotic-inactivating enzymes, like β-lactamases (1). It also possesses acquired and adaptive resistances, leading to high morbidity and mortality rates among patients. Moreover, misuse and excessive use of antibiotics has accelerated the development of multidrug resistance for P. aeruginosa. It is now listed in the “critical” category among bacterial pathogens by the World Health Organization (WHO), meaning that there is an urgent need for innovative research and the development of new antibiotics in this field (2).
In addition, the antimicrobial resistance developed by P. aeruginosa towards food preservatives poses an intractable challenge in food industries, and causes enormous economic loss. P. aeruginosa biofilm, tough to be eradicated, adheres to surfaces that are either biotic or abiotic, thus is commonly found in the spoiled seafood, chill-stored foods, milk, meat products, and water used for food processing (3). Microbial biofilms of bacteria attach to the surface of food processing equipment. Meanwhile, environmental factors in food industries facilitate the formation and development of bacterial biofilms. Food matrix composition, temperature, hydrodynamic effects, oxygen concentration, and microbial interactions all contribute to the production of biofilms. Owing to proteolytic and lipolytic activities of P. aeruginosa, food safety and quality are significantly threatened. Altogether, effective antibiotic agents are urgently needed worldwide for both healthcare and food industry.
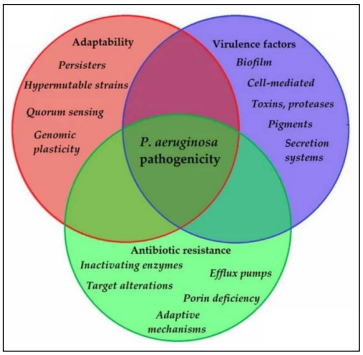
Figure 1: Main components of P. aeruginosa pathogenicity (14)
EOs, natural products usually extracted from plants, are known for being major components of perfumes, providing the fragrances of food and cosmetics products. EOs have been put into medical use for centuries and have huge potential in being alternatives for antibiotics in P. aeruginosa infection treatments. By compromising cell membrane integrity, inhibiting efflux system’s activities, and decreasing production of elastase, pyocyanin, and swarming motility (important indicators of quorum sensing operon in P. aeruginosa), EOs alleviate the virulence factors of P. aeruginosa. Unlike conventional antibiotics, P. aeruginosa is less likely to gain resistance to EOs. Furthermore, EOs show synergy effects when using with conventional antibiotics (3). However, the characteristics of EOs, such as lipophilicity, high volatility, and easy decomposition after exposure to environmental factors (4) preclude the feasibility of utilizing EOs to patients or industrial use. Multiple lipid nanoparticles (MLC), which are developed from simple lipid carriers and nanostructured lipid carriers (NLC), may stabilize EOs and solve the abovementioned problem.
Studies to explore the potential of MOs in treating P. aeruginosa are available but limited. Our review summarizes the mechanism of P. aeruginosa pathogenicity, EOs' biological functions (especially antimicrobial), the interaction between EOs and P. aeruginosa and incorporating innovative technology into EOs.
P.aeruginosa
Pseudomonas aeruginosa, belonging to the family Pseudomonadaceae, is a gram-negative bacterium that often acquires multi-drug resistance. It is one of the leading causes of infections in intensive care units (ICU) and can cause both acute and chronic infections, dramatically increasing the morbidity and mortality rate among patients (5). The strong adaptability and metabolic flexibility of P. aeruginosa stem from its large variety of virulence factors and its antibiotic resistance (6). Seven virulence mechanisms of gram-negative bacteria, most of which are present in P. aeruginosa are showed in Figure 2A and P. aeruginosa can lead to multiple types of infections including pneumonia, osteomyelitis, implant-associated infections, skin burn infections, urinary tract infection, and catheter-related infections (Figure 2B).
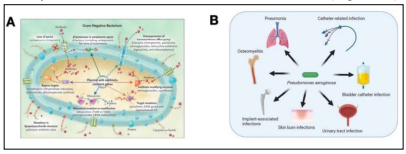
Figure 2: Schematic representation of P. aeruginosa virulence mechanisms (A) and main infections caused by P. aeruginosa (B) (5, 15).
P. aeruginosa is often multidrug-resistant, acquiring resistance through complex mechanisms, including intrinsic, acquired, and adaptive resistance (1). Intrinsic resistance includes low permeability of the outer membrane, efflux pumps that expel antibiotics from the host cell, and the production of enzymes that inactivate antibiotics. During chronic infections, P. aeruginosa evades recognition by the host’s innate immune system by forming dense biofilm structures on both surfaces of host tissue and interfaces of medical implants. The dense biofilm also decreases the permeability of antibiotics, allowing the bacteria embedded in the structures to be virtually impervious to antibiotics. Efflux pumps can pump out antibiotics, and reduce the intracellular concentration of antibiotics (7). Acquired resistance is developed through gene mutations or horizontal transfer of resistance genes. Adaptive resistance is the mechanism to prevent antibiotics from entering bacteria. One example is P. aeruginosa forms biofilms which operate as diffusion barriers.
To treat P. aeruginosa infections, most antibiotics must have the ability to penetrate the cell membrane of bacterium. As a selective barrier preventing antibiotics penetration, the outer membrane of P. aeruginosa is an asymmetric bilayer of phospholipid and lipopolysaccharides, with porins that form β-barrel protein channels. In general, the porin family can be divided into four classes: efflux porins (crucial part of efflux pumps), specific porins (specific sites to bind particular molecules), gated porins (ionregulated outer membrane proteins responsible for uptake of ion complexes), and non-specific porins (slow diffusion of most small hydrophilic molecules (1). Among them, OprF, the predominant porin of P. aeruginosa, plays the role of non-specific uptake of ions and saccharides.OprF channels are mostly closed, with less than 5% open, leading to the low permeability of P. aeruginosa compared to other bacteria. In addition, OprD, a specific porin involved in β-lactam antibiotic uptake, is the smallest porin in P. aeruginosa and sometimes is found absent in P. aerginosa. The absence of OprD can increase its resistance to antimicrobials. The outer membrane permeability of P. aerginosa is 12 to 100-fold lower than that of E. coli (1).
Efflux systems, pumping toxic compounds out of the bacteria are critical in the development of antimicrobial resistance. The resistance-nodulation-division (RND) family of efflux pumps dominates P. aeruginosa antibiotics resistance among the five families: resistance-nodulation-division (RND) family, major facilitator superfamily (MFS), ATP-binding cassette (ABC) superfamily, small multidrug resistance (SMR) family, and multidrug and toxic compound extrusion (MATE) family (1). Some strains of P. aeruginosa have been shown to overexpress several efflux pumps, which broadens bacterial antibiotic resistance and aids in the development of multidrug resistance.
Essential oils (EOs) are natural oils, usually extracted from plants by distillation. Normally, they have a small molecular weight and are relatively water-soluble. However, EOs, mainly containing terpenes hydrocarbons and oxygenated compounds, have a high solubility in alcohol (Figure 3). Terpenes are odorous products, due to the presence of double bonds and ring structures, acquiring the characteristic fragrances of the plants or the source from which they are derived. Histological records of EOs can be dated back to as early as 500 B.C., when Greeks documented EOs adopted from Egyptians. EOs have been alternatives for medicines due to a variety of biological functions, including antioxidant, antiviral, anti-mutagenic, anticancer, and antimicrobial. EOs can prevent cells from being oxidized by free radicals, such as reactive oxygen species. Reactive oxygen species are active molecules that lead to oxidative diseases, such as cancer and Alzheimer’s disease. Meanwhile, EOs can function as antifungal medicine by attacking the life cycle of molds. They can also be used along with chemotherapy to prevent adverse effects from chemotherapy (8). All these features make EOs a potential and innovative agent in healthcare arena.
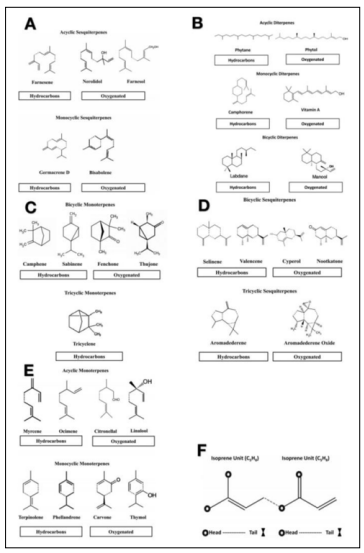
Figure 3: Diverse chemical structures of Essential Oils. A-E, EOs from different natural plants. F, Isoprene Units, building blocks of EOs (8).
Three methods are most used to extract EOs from plants: expression, distillation, and solvent extraction. Expression is the first method developed. Peels of plants are pressed mechanically to obtain largescale production. Distillation starts with placing raw plant material, such as flowers, over water in an alembic (distillation apparatus). As water boils, water vapor passes through the plant material, and volatile compounds in the plant's vapor rise with water steam. Vapor flows across a coil, condensing back into liquid. After the process, the essential oil is separated from the mixture of oils and plant water essence. In general, there are three types of distillation: water distillation, water and steam, and steam distillation. Solvent extraction is used to extract the essence from plants with delicate structures or those containing very little oil (Figure 4).
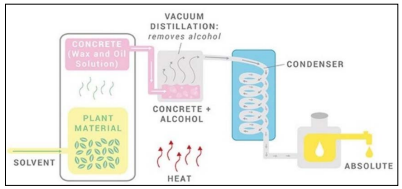
Figure 4: The process of solvent extraction used in perfumery (New directions aromatics: https://www.newdirectionsaromatics. com/blog/articles/how-essential-oils-are-made.html).
EOs are widely used in cosmetics, perfumes, food, drinks, air freshers, and other products as flavored fragrances. They also have huge potential for healthcare. The highest antibacterial activity has been seen in EOs with aldehydes or phenols as main components, including cinnamaldehyde, citral, carvacrol, eugenol, and thymol. The phenolic components in EOs are thought to be the source of the antimicrobial characteristic (9). EOs also induce relaxation in aromatherapy. Furthermore, researchers have discovered the antimicrobial effects of EOs by inhibiting pathogens. EOs target efflux pumps, biofilms, and quorum sensing (QS). Due to their lipophilic characteristics, EOs have high permeability and can penetrate through the cell walls and cell membranes easily. Reacting with chemicals such as fatty acids and phospholipids, EOs can denature cellular proteins, compromise membranes, which lead to the leakage of the cellular contents, and inhibit the activities of the proton pump, leading to the cell death eventually (8).
Various bacterial pathogens are susceptible to the broad-spectrum inhibitory effects of EOs. Because EOs’ lipophilic nature, they can easily pass through cell membranes and cell walls. Interaction of EOs components with bacteria components, such as polysaccharides, fatty acids, and phospholipids, interferes proton pump activity, damages membrane integrity, and causes cellular contents leak and eventually cell death. Denaturation of cellular proteins is one of the key mechanisms of action (Figure 5).
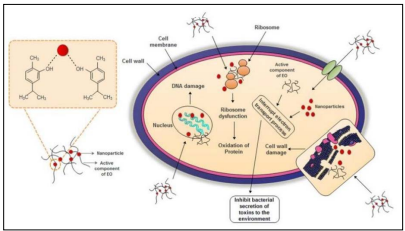
Figure 5: Application of EO as antimicrobials (16)
Additionally, EOs act in synergy with antibiotics. This finding is remarkable because the antibiotics that P. aeruginosa resistant to can be used for treatment along with EOs.
EOs act as efflux pump inhibitors (EPI) to eliminate P. aeruginosa pathogenicity. By interfering with the regulatory processes and the expression of the efflux pumps, EPIs stop antibiotics from being extruded. In a recent study, researchers used Satureja khuzistanica (S. khuzistanica) essential oil (SKEO), which primarily contains thymol and carvacrol (chemicals that can damage bacteria) and tested its inhibition effects on efflux pumps. The study shows that these chemicals indirectly inhibit the gene expression of efflux pumps. The minimum inhibitory concentration (MIC) was measured to test the antimicrobial property of SKEO. By using a checkerboard synergy assay, the impact of SKEO in combination with gentamicin or norfloxacin was also assessed. This study demonstrates that SKEO's antibacterial efficacy against P. aeruginosa strains is dose-dependent (Figure 6). The data is classified as synergism and indifference based on the fractional inhibitory concentration index (FICI) value (10). The effectiveness of SKEO and its synergy effects are consistent in different strains.
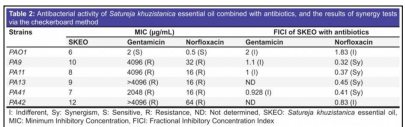
Figure 6: The results of antibacterial activity from SKEO and SKEO combined with gentamicin or norfloxacin (10).
Biofilms play an essential role in P. aeruginosa pathogenicity. Thus, inhibiting the development of biofilms is important to eliminate P. aeruginosa. EOs inhibit biofilm production and bacterial metabolic activities in biofilm. In a study testing antibacterial activities of Lemon Oils in 2021, different essential oil samples of Lemon Oils were all able to inhibit biofilm production in a dose - dependent manner (Figure 7). Moreover, the observed inhibition effect of the major compound limonene was minimal, indicating the possibility of existing synergistic interaction between the compounds of Lemon Oils (3).
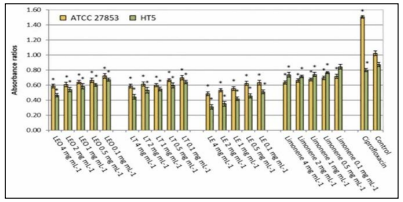
Figure 7: Specific biofilm formed by Pseudomonas aeruginosa strains without (control) and in the presence of ciprofloxacin (5 µg/ml) or different lemon oils concentrations (0.1-4 mg/ml). (LEO: Lemon essential oil, LF: Lemon terpenes, LE: Lemon essence. Asterisk indicates significant differences compared to the respective control (Tukey’s multiple range test, p < 0.05) (3)).
An in vivo study in mice model was conducted to test EOs treating burn wound infections (11). In the experiment, mice had free access to food and water and were kept in the same conditions for more than three days. Burn injury was produced using the hot water method. Fifteen mice were then divided into five groups with different treatments as follows: group 1, no treatment; group 2, cinnamon oil; group 3, NLC-cinnamon oil colloid; group 4, NLC blank gel; and group 5, NLC-cinnamon oil gel. Only group 5 was treated with EOs. Wounds were evaluated daily by checking the wound surface and edge contraction. Group 3 had similar healing progress to group 5 and group 5 showed a more complete induration of new skin growth and smooth wound edge with no signs of infection (Figure 8). The infected wounds in group 1 looked like a chronic erythematous infectious lesion with no improvement. The skin lesions in group 2 had an edematous, scarred surface with a dispersed wound edge, which may be caused by the irritant effects of the cinnamon oil when it was diluted in a carrier oil and administered directly to the open wound. The enlarged area of wound in group 2 suggests the unfeasibility of directly applying EOs to the skin. Group 5 shows the lowest burn contraction area (Figure 9), which was the only group treated with EOs. Overall, this study demonstrated EOs’ ability to inhibit microorganisms and promote the wound healing process.
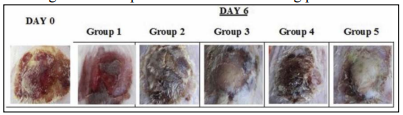
Figure 8: Images of original burn wound (day 0) and after treatment on day 6. Group 1: no treatment, group 2: cinnamon oil, group 3: NLC-cinnamon oil colloid, group 4: NLC blank gel, and group 5: NLC-cinnamon oil gel (11).
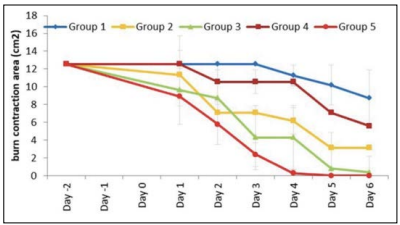
Figure 9: Burn area contraction (cm2 ) of tested groups. Group 1: no treatment; group 2: cinnamon oil; group 3: NLC-cinnamon oil colloid; group 4: NLC blank gel, and group 5: NLC-cinnamon oil gel (p<.0001) (11).
Although EOs remain an effective approach treating P. aeruginosa infections, their stability and toxicity pose difficulties in real-life application. After being extracted from plants and exposed to environmental factors, EOs quickly decompose. Injection of EOs is not feasible in clinical practice due to its lipophilicity and high volatility. The costs of transportation and storage of EOs can be high, and their instability also contributes to severe economic loss. Additionally, EOs are potentially harmful and hazardous. EOs can be combustible and phototoxic, causing skin dermatitis, chemical burns, or even death (12). The composition of EOs is complex, including antimicrobial components and chemicals that may promote P. aeruginosa activities. In addition, A variety of factors such as geographical and environmental conditions, for example, temperature, rainfall, altitude, and hours of sunshine, influence plants' growth, thus make EOs’ composition subtly different.
To increase EOs' water solubility, bioavailability and physicochemical stability during processing and storage, nanoencapsulation has been proposed as a potential method. BenKhalifa, R. and et al. demonstrated the feasibility of loading EOs into multiple lipidnanoparticles (MLNs) (4). It has been suggested that using lipid carriers is an alternative method for improving the antibacterial properties of many antimicrobial drugs. In fact, it has been shown that the lipid layers of these systems can bind to bacterial outer membrane, enabling the direct delivery of encapsulated medications. Second-generation lipid nanoparticle made up of a mixture of solid and liquid lipids encased in a surfactant is called a nanostructured lipid carrier (NLC). MLN was developed from NLC in respect of NLC’s hydrophobic nature. Instead of incorporating hydrophilic agents into NLC formulations, lipophilic agents were incorporated, giving birth to MLN (Figure 10). In the study, the packaging of Rosemary EO (REO) in nanoscale particles significantly increased its antibacterial efficacy against all tested strains of P. aeruginosa, according to the Antimicrobial Susceptibility Testing results (4).
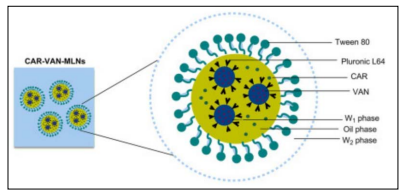
Figure 10 : The structure of Multiple Lipid Nanoparticles (17).
Due to the intricacy of its chemical components, EOs have been shown in multiple studies to not only have potent antibacterial action, but also work synergistically with conventional antibiotics. Magi, G. and et al. tested the antibacterial efficacy of various EOs and carvacrol against erythromycin-resistant Streptococcus pyogenes [Group A streptococci (GAS)] isolated from children with pharyngotonsillitis in Italy, both on their own and in conjunction with erythromycin (13). They found that EOs acted in synergy with erythromycin against erythromycin-resistant GAS, suggesting the possible re-use of erythromycin in combination with EOs in the treatment. This marks an alternative strategy in fighting antimicrobial-resistant bacteria. After discovering more about the mechanisms, multiple combinations can be developed. Allowing the re-use of previously resisted drugs dramatically is fiscally efficient. The synergy effects of EOs with antibiotics establish a promising approach to easing the obstacles that AMR brings.
This review depicts the antimicrobial effects of Essential Oils on P. aeruginosa. Existing resources in this area are mostly in vitro and are limited. On the other hand, EOs have an enormous potential to act as alternatives to antibacterial treatments. The variety of EOs is inexhaustive due to the diversity of plants that EOs are extracted from. Researchers should find similarities among EOs and pinpoint the mechanisms of components that are efficient against bacteria. Additionally, further investigating EOs’ synergy with antibiotics opens a novel approach from the old loop of developing new antibiotics against bacteria, which then quickly gain resistance. However, due to the immaturity of the area in EO applications, direct application to patients in the short term may be risky. Hence, developing EOs’ industrial usages will not only benefit industries, but also guide clinical managements.
The rapid development of AMR in P. aeruginosa has cost financial loss in food industries, increased morbidity and mortality, extended length of treatment, and increased financial burden in healthcare management. An innovative approach to exploring EOs’ antimicrobial and synergy effects has contributed to alleviate the problem.
Though EOs’ complexity and delicacy add difficulties to reallife applications, EOs, loaded in MLNs, effectively attenuate the pathogenicity of P. aeruginosa by compromising membrane integrity, alleviating virulence factors, and inhibiting efflux pumps’ activities. Future translating experiments from in vitro to in vivo are necessary to focus on mechanisms, potential risks and sideeffects, and applications in both industrial and clinical settings safely.
