Author(s): <p>Sávio Samuel Feitosa Machado, Wladia Gislaynne de Sousa Tavares, Isabella Sá de Quental, José Maurício Pereira Lopes, Allana Maria Garcia Sampaio Cruz, Pedro Walisson Gomes Feitosa and Modesto Leite Rolim Neto</p>
Introduction: The pandemic of the new coronavirus (SARS-CoV-2) has unfolded at a remarkable speed, presenting unprecedented challenges for society and for health systems. The experience with coronavirus infections during pregnancy is still limited, requiring analysis regarding the impact of SARS-CoV-2 on the health of pregnant women and newborns. We aimed to report a case of fetal death of a pregnant woman with COVID-19 and SARS-CoV-2 identified in the placenta with morphological repercussions.
Case Report: Primigravida, 39 years old, gestation initially twinned, evolved non-evolution of the second embryo, verified in ultrasound evaluation in the first trimester of pregnancy. In the course of 35 weeks, she reported watery diarrhea, without blood or mucus, with progressive worsening, about 10 to 12 episodes in 24 hours, associated with diffuse colicky abdominal pain. She denies having other symptoms. 48 hours after the onset of diarrhea, the pain worsened, radiating bilaterally into the lumbar region, in addition to prostration and asthenia, seeking emergency care at a maternity hospital. Physical examination revealed active labor (9 cm dilation), rupture of ovular membranes and lack of fetal vitality by cardiac auscultation and evaluation of fetal movement. A cesarean section was performed, in which fetal death was confirmed. Soon after the procedure, still in the delivery room, a naso-oropharyngeal swab was collected from the patient, and SARS-CoV-2 was detected through RT-PCR technique.
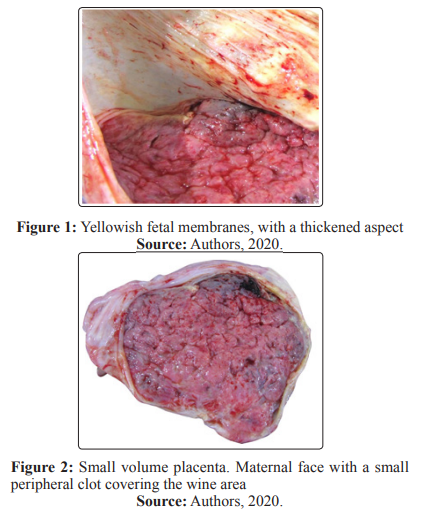
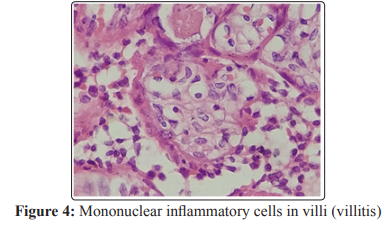
Conclusion: The maternal surface of the placenta had an area of previous infarction and an area of recent infarction, peripheral, making up about 20% of the organ and the placental cuts showed white bundles. The fetal membranes had thickened and yellowish areas. Histopathological examination of the placenta showed diffuse villitis, characterized by lymphoplasmacytic infiltrate, in addition to intervillositis with areas of intense intervillous fibrin deposition. Swab collection was performed from the placental cut surface for detection of SARS-CoV-2 by RT-PCR, which was positive. The naso-oropharyngeal swab of the fetus was negative.
After the start of the notifications of infections by a new coronavirus in Hubei, China, severe cases of pneumonia with clinical characteristics of viral infection were recorded in different parts of the globe. Thus, the World Health Organization (WHO) declared, on March 11, 2020, COVID-19 a pandemic, referring to a public health emergency of international interest due to this SARS-CoV-2 infection, not because of the severity of the disease, but due to the rapid geographical spread that it has presented [1]. Researches are developed around the world in order to assess the epidemiological and clinical characteristics of this disease, the COVID-19, caused by the SARS-CoV-2 virus, a member of the Coronaviridae family, which causes, in humans, diseases with variable clinical features, from asymptomatic to cases with severe gravity [2].
Since the first reports of COVID-19, an increasing number of pregnant women and children affected is notified, highlighting the interest of obstetricians and pediatricians in the understanding of the phylogenetic and pathogenic aspects of the virus as well as the natural history of the disease and clinical outcomes related to infection and its therapy [3]. Schwartz, analyzed the literature describing 38 pregnant women with COVID-19 and their newborns in China to assess the effects of SARS-CoV-2 on mothers and babies, including clinical, laboratory and virological data and transmissibility of the virus from the mother to the fetus [4]. Schwartz, reveals that, on these 38 pregnant women, COVID-19 did not lead to maternal deaths and there were no confirmed cases of SARS-CoV-2 intrauterine transmission from affected mothers to their fetuses [4].
The analysis of the clinical course of the disease in pregnant women can assist in the development of appropriate protocols regarding recommendations to pregnant women and to the perinatal care. The study of the relationship between the delivery method and the risk of transmission from mother to the fetus are incipient and require deepening. The set of current evidence does not suggest that pregnant women are more susceptible to disease and to their respiratory complications, in contrast to the diseases caused byother strains of coronavirus, SARS and MERS [5].
However, adverse perinatal outcomes have been reported, including increased risks of miscarriage, pre-eclampsia, premature birth and stillbirth. In this way, the histopathological study of placental tissue, in addition to relevant information about the health of the mother and the fetus, can contribute to increasing the understanding of this public health issue [6]. This study aims to report a case of fetal death in a pregnant woman with a current infection with SARS-CoV-2 detected by real-time polymerase chain reaction (RT-PCR) in nasopharyngeal swab, as well as to discuss the relationship of maternal infection by COVID -19 with placental histological changes and possibility of fetal infection.
Primigravida, 39 years old, gestation initially twinned, presented non-evolution of the second embryo, verified in ultrasound evaluation in the first trimester of pregnancy. During pregnancy, she underwent a cervical cerclage due to short cervix. Routine serological tests in the first trimester for rubella and cytomegalovirus showed positive IgG and negative IgM; as for toxoplasmosis, both IgG and IgM showed negative results. Over the course of 33 weeks, she was admitted to the hospital with a diagnosis of pyelonephritis, reporting myalgia, asthenia, fever and with a positive urine culture. During the same hospital admission, the patient started to present mild flu-like symptoms, referring rhinorrhea, odynophagia and dry cough, with no others respiratory complaints. According to the patient, the symptoms persisted for 3 days. After 7 days of antibiotic therapy, the patient was discharged.
In the course of 35 weeks, she reported watery diarrhea, without blood or mucus, with progressive worsening, about 10 to 12 episodes in 24 hours, associated with diffuse colicky abdominal pain, with no other symptoms. 48 hours after the onset of diarrhea, the pain worsened, radiating bilaterally into the lumbar region, in addition to prostration and asthenia, seeking emergency care at a maternity hospital. Physical examination revealed active labor (9 cm dilation), rupture of ovular membranes and lack of fetal vitality by cardiac auscultation and evaluation of fetal movement.
The patient denied fever and respiratory symptoms, but reported contact with her spouse who was admitted to another hospital with pneumonia and suspected COVID-19, diagnosis that was later confirmed by RT-PCR. The patient refused to continue vaginal delivery and was led to a cesarean section, in which were confirmed fetal death and lack of amniotic fluid.
Soon after the procedure, still in the delivery room, a nasooropharyngeal swab was collected from the patient, and SARSCoV-2 was detected through RT-PCR technique. The placenta and the fetus were sent to the local Death Verification Service for examination. Before the autopsy, rapid tests for HIV, syphilis, dengue, hepatitis B and C were performed in the fetus; all negative. The fetal body weighed 1990g, being below the expected weight for gestational age. However, it showed developmental aspects compatible with gestational age, presenting female genitalia, skin with signs of maceration and absence of malformations. The lungs showed wine color, compact consistency and there was an apparent hepatomegaly. The placenta had an irregular shape, weighting 245 g, below the percentile 3 to Thompson et al., about one eighth of fetal weight, and measured 17.5 x 14.5 x 1 cm [7]. The maternal surface of the placenta had some lobes with little distinct borders, presenting an area of previous infarction and an area of recent infarction, peripheral (Figure 1), making up about 20% of the organ The fetal membranes had thickened and yellowish areas (Figure 2) and the umbilical cord had peripheral implantation, with three vessels. Moreover, the placental cuts showed white bundles (Figure 3)
Histopathological examination of the placenta showed diffuse villitis, characterized by lymphoplasmacytic infiltrate, in addition to intervillositis with areas of intense intervillous fibrin deposition. The fetal membranes presented areas with neutrophilic inflammatory infiltrate and necrosis foci. The analysis of histological sections of the lung was impaired due to autolysis, but the presence of a mixed inflammatory infiltrate was noticeable. Swab collection was performed from the placental cut surface for SARS-CoV-2 detection by RT-PCR, which was positive (Figure 3). The naso-oropharyngeal swab of the fetus was negative.
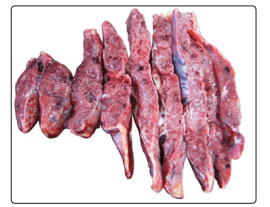
Figure 3: Placental section surface (where the swab was collected) showing sparse white bundles and peripheral area suggestive of old infarction.
The patient and her husband signed the Informed Consent Form (ICF) authorizing researchers to analyze and publish their clinical history in scientific circles.
This report presents the placental anatomical-pathological analysis of a patient who evolved with COVID-19 and fetal death in Brazil. An evidence of significant interest in studies regarding this topic are the recurrent description of poor maternal vascular perfusion, such as areas of placental infarction and diffuse villitis. These evidences are commonly associated with oligohydramnios, fetal growth restriction, premature labor, and are also seen in maternal hypertensive disorders, including gestational hypertension and preeclampsia [8-11]. Surprisingly, the patient in this case did not present any type of hypertensive syndrome during the entire pregnancy.
Since SARS-CoV-2 is a virus, induction of inflammation is expected. Chronic villitis, as seen in the reported case, can be directly caused by some viral infections, such as Zika Virus [12]. The chronic villitis also shows seasonality, suggesting its relation to circulating viruses causing a direct infection or loss of tolerance [13]. The reported pregnant patient with COVID-19 presented negative serologies for HIV, syphilis, dengue, hepatitis B and C, Cytomegalovirus and Toxoplasmosis. The evidence of villitis in the analyzed case is shown in Figure 4.
Moreover, in this case report, the maternal surface of the placenta presented one area of previous infarction and one area of recent infarction, making up about 20% of the surface, added to whitish spots in cutting areas. Patients with COVID-19 showed a significant increase of intervillous thrombus. These intervillous thrombus are usually considered incidental findings, but are associated with maternal hypertensive disorders or coincident infarcts [14]. In studies that relate an increase of thrombotic and thromboembolic disorders in COVID-19, it is showed that they may represent deposition of thrombus in response to the virus [15,16].
In line with the findings of our report, Chen et al. describes placental findings in three women with COVID-19 [2]. The authors showed increase of perivillous fibrinoid material deposition in all three placentas, multiple villous infarcts in a placenta and chorioangioma in another case. Two others case reports of miscarriage in women with COVID-19 in the second trimester of pregnancy are highlighted. Baud et al., describe a case in which the placenta showed acute inflammation and increased perivillous fibrin [17]. Similar findings in the reported case are shown in Figure 5. Hosier et al. report evidence of histiocytic intervillositis with viral infection and spike protein in the syncytiotrophoblast, demonstrated by immunohistochemistry, suggesting a possible association of these placental events with SARS-CoV-2 [18]. Algarroba et al., visualized, through electron microscopy, coronavirus virions invading syncytiotrophoblast in placental villi, featuring direct evidence of SARS-CoV-2 virus invasion in placental tissue and placental infection associated with SARS-CoV-2 [19].

Shanes et al., described placental findings from sixteen patients with SARS-CoV-2 infection [20]. The authors did not suggest any pathognomonic findings. In the study of the placental changes that SARS-CoV-2 may trigger, out of the sixteen placentas of patients with SARS-CoV-2 (14 term deliveries, 1 premature at 34 weeks and 1 abortion at 16 weeks), five placentas were small for gestational age, as described in our report. Poor maternal vascular perfusion was also evidenced in 12 out of 15 studied placentas, as well as peripheral villous infarcts, villous agglutination and accelerated maturation of the villi, placental arteriopathy including atherosis and fibrinoid necrosis of maternal vessels and vessel wall hypertrophy of membrane arterioles.
Among the cases described by Shanes et al., the first case showed slightly increased perivillous fibrin [20]. The SARS-CoV-2 nucleic acid test was negative in the fetus, but positive in the placenta. Bacterial culture and PCR were negative, leading the authors to suggest that the acute inflammatory response is related to SARSCoV-2. In our case, we also report intervillositis with areas of intense intervillous fibrin deposition. Low-stage inflammation is common in labor and the investigation of bacteria is often negative [21]. A greater case series is needed to estabilish the relationship between SARS- CoV-2 and acute inflammation. However, current evidence supports the possibility that vertical transmission of the virus is uncommon, with placental changes, if caused by COVID-19, being related to maternal infection and inflammation, not to fetal infection. Thus, Li et al., point out that the SARS-COV-2 receptor in cells, the angiotensin-converting enzyme (ECA2), has a deficient expression in the different types of cells belonging to the placental interface, emphasizing the low risk of transmission of the virus in the intrauterine environment from mother to child [22].
In an analysis similar to our report, Penfield et al., (2020) analyzed 11 placental or membrane swabs that were sent for SARS-CoV-2 detection after delivery. Out of these, three were positive for the virus, all being from women with moderate to severe disease at labor. The authors claim that their findings could represent contamination of maternal blood, amniotic fluid or SARS-CoV-2 infection. In Penfield et al., all newborns tested negative in nasopharyngeal swab collected in the first five days of life [23]. In our case report, we also evidenced the presence of SARS-CoV-2 by RT-PCR on placental swab, even though the pregnant woman had only a mild presentation of the disease and a negative nasooropharyngeal swab of the fetus.
Thus, we infer that the SARS-CoV-2 infection may be related to the appearance of serious pregnancy complications, being associated with an increased risk of fetal death. Evidence in the literature regarding placental repercussions of COVID-19,as well as vertical transmission of the infection is still scarce, and studies with this approach are needed in order to broaden the understanding of this new urgency in public health with specific data on maternal-child health. It is highlighted that, in the presented case, chorioamnionitis (probably associated with maternal urinary tract infection days before birth) led to premature rupture of membranes and consequent oligohydramnios; this fact, in addition to the poor placental perfusion, which has been frequently reported in pregnant women with COVID-19, caused fetal death. Limitations of this work were the impossibility of RT-PCR analysis of fetal samples and the complementation of other techniques of morphological study for the placental tissue.
Brazilian National Council for Scientific and Technological Development (CNPq) - institution linked to the Brazilian Department of Science, Technology and Innovation to encourage research in Brazil.
The Brazilian National Council for Scientific and Technological Development (CNPq) - institution linked to the Brazilian Department of Science, Technology and School of Medicine/ Universidade Federal do Cariri -UFCA.
