Author(s): Kunal Gharat, Vikas Jha*, Joshua Koli, Ashish Jhangiani, Vrushali Dhamapurkar, Diksha Poojari, Omkar Parulekar, Simeen Rumani Dhanashree Kulabkar and Bhakti Salekar
A quarter of the world’s population is thought to be latently infected with Mycobacterium tuberculosis, creating a vast pool of possible future illnesses. Combined with the rising prevalence of multi-drug resistant Mycobacterium tuberculosis (Mtb) strains, TB poses one of the biggest challenges for world health, and the TB epidemic can only be eradicated with a new and more effective vaccine strategy. Since the Bacille Calmette-Guérin (BCG) vaccine is the only one used to treat tuberculosis (TB), Reverse vaccine technology aims to accelerate the development of subunit vaccines by identifying specific proteins in a pathogenic bacterial proteome that may be protective antigens. This approach was employed on four extracellular proteins namely Secreted fibronectin-binding protein C antigen 85-C, PE-PGRS family protein, Invasion protein RipA, and Invasion protein RipB. The shortlisted proteins were subjected to B-cell, CTL, and HTL epitope prediction. To further identify the most promising epitopes, specialized filtering techniques were employed to narrow down CTL epitopes that were non-allergenic, non-toxic, and antigenic as well as B-cell epitopes that produced antibodies. Similarly, HTL epitopes that generate IFN- γ but not IL-10 were selected. The analysis revealed invasion protein RipA as a potential candidate for Mycobacterium tuberculosis CCDC5180 immunization.
Tuberculosis, Malaria and Acquired immune deficiency syndrome (AIDS) are the 3 major infectious diseases that cause a significant disease burden with high rates of morbidity and mortality [1]. There were an estimated 8.6 million incident cases of TB worldwide in 2012, which is equal to 122 cases per 100,000 individuals [2,3].
TB typically manifests as a pulmonary disease transmitted by droplet inhalation, with symptoms including a persistent cough, fever, and night sweats [4]. Individuals with healthy immune systems can often suppress the disease, remaining asymptomatic but infected. Immunocompromised patients who are unable to mount an immune response sufficient to suppress symptoms face difficulties [5]. The slow growth rate of M. tuberculosis, its complex pathogenesis, and its ability to remain dormant all pose significant challenges to the development of effective anti-TB treatments [6]. As a result, TB vaccination remains a top priority. The finding of essential mycobacterial virulence genes has been extremely beneficial Extensive research on virulence genes, made possible by the use of transposon mutant libraries and a range of in vivo screening approaches, has shed light on the ability of the bacilli to survive to live and persist in their hosts [ 7, 8].
According to WHO survey data from around the world, 0.6 million cases of multidrug-resistant tuberculosis (MDR-TB) were reported, with approximately 0.24 million deaths [9]. MDR-TB strain growth leads to insufficient or irregular antibiotic intake and limited TB treatment. Individuals with low immunity are more resistant to infection and unable to respond to the immune system effectively. As a result, more efforts are needed to advance the development of new TB vaccines [10]. Cell immunity (CD4+ and CD8+ T cells) accounts for the majority of the immune response to Mtb. Once activated, both CD4+ and CD8+ T cells secrete cytokines that cause an immune response. CD8+ cells also mediate cytotoxicity and lysis of infected cells. An Effective T cell responses are required for tuberculosis elimination. Complex pathogenesis, slow Mtb growth, and dormant ability are critical tasks in developing effective TB therapies.
Researchers have used subtractive genomics and reverse vaccinology to discover promising therapeutic targets and vaccine candidates against a variety of pathogenic microbes. Reverse vaccine technology seeks to accelerate the development of subunit vaccines by determining which proteins in a pathogenic bacterial proteome are possible protective antigens [11]. It was initially proposed in 2000, based on the discovery of new meningococcal vaccine candidates from a Neisseria meningitidis serogroup B strain’s genomic sequence. Over the last decade, the use of reverse vaccinology has revolutionized how vaccine development is conducted, and this new age of vaccine research has been labelled a renaissance of vaccinology [12]. The fundamental benefit of reverse vaccinology over conventional vaccinology is the time and cost savings associated with identifying prospective vaccine candidates, as there is no need to culture the bacteria [13]. Furthermore, reverse vaccinology can find all potential protective protein antigens for a bacterial species as opposed to merely the most abundant antigens that can be identified from bacterial cultures using a conventional method. The accessibility of whole-genome sequencing has transformed the approach to vaccine development and led us to adopt fresh perspectives.
Ali et al. applied a subtractive genomics approach to discover drug targets and vaccine candidates against Borrelia burgdorferi B31, Ehrlichia chaffeensis [14]. Arkansas et al. used a reverse vaccinology approach for designing vaccines against Pseudomonas aeruginosa [15]. In addition, they developed a chimeric vaccine against Acinetobacter baumannii using a combination of subtractive genomics and reverse vaccinology technique. Arshad Dar et al. developed a multi-epitope vaccine against Klebsiella pneumoniae using immunoinformatics tools [16]. Furthermore, they performed molecular docking studies using Toll-like receptors (TLR) to validate the vaccine design, demonstrating a strong contact between their design and TLR. Bibi et al. recently used a reverse vaccinology technique to find promising M. tb H37Rv vaccine candidates. They developed a multi-epitope vaccine using four antigens: Rv2608, Rv2684, Rv3804c, and Rv0125. The construct was further evaluated by docking it with TLR3 using four online servers: ClusPro, HADDOCK, FireDock, and HawkDock, with a score ranging from -30 to -42 kcal/mol [17]. The objective of this study was to identify potential vaccine candidates from the whole proteome of of M. tb CCDC5180 by using online databases to uncover extracellular crucial proteins by subtractive proteomics approach. The transmembrane helices and the antigenicity of the protein were identified. Both epitope mapping and the construction of a multi-epitope vaccine was achieved. Further, the physicochemical characterization of these multi-epitope vaccines was carried out.
The essential proteins shortlisted for the study were selected from the DEG database (http://origin.tubic.org/deg/public/index.php ). The proteins were chosen based on their importance and location within the cell. These proteins were discovered and used as a resource to find potential vaccine candidates.
Identifying the protein’s antigenic properties is a vital stage in the vaccine development process. Determining the antigenic nature of a protein is a time-consuming and expensive procedure, but the proteomics revolution in bioinformatics has made this determination incredibly simple and affordable. The antigenic nature of the shortlisted extracellular proteins was computed using VaxiJen 2.0 web server ( http://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html ) [18]. A threshold was set at 0.4 and proteins above this threshold were considered to be potential antigens.
Secretory proteins can play a significant role in enhancing bacterial pathogenicity, by enabling adhesion to host cells and impairing their defense mechanism [19]. The secretory nature of the antigenic extracellular proteins was identified using the Signal-BLAST online server ( http://sigpep.services.came.sbg.ac.at/signalblast.html ). Antigenic proteins were labelled as signal peptides based on four criteria: sensitivity, prediction, specificity and cleavage location [20].
The trans-membrane integral inner membrane proteins (IMPs) span the plasma membrane and contain α-helices. This is helpful in predicting potential vaccine candidates as proteins with less than two membrane helices are easy to clone and purify [21]. TMHMM2.0 (Trans-membrane helices hidden Markov model for topology prediction) ( http://www.cbs.dtu.dk/services/TMHMM/ ) was used to calculate the number of transmembrane helices. TMHMM is a membrane protein topology prediction method based on a hidden Markov model that accurately predicts transmembrane helices and distinguishes between membrane and soluble proteins. Similarly, proteins with molecular weight less than 100 kDa were preferred for the ease of purification. The ProtParam tool ( https://web.expasy.org/protparam/ ) in the ExPASy server was used to estimate the molecular weight of these extracellular proteins [22].
Continuous/linear and discontinuous epitopes are the two existing forms of B cell epitopes. The linear B-cell epitopes are 16-amino acid sequences with a short antigenic length that can cause the development of particular antibodies. Antigenic proteins having a molecular weight of less than 100 kDa and no more than two transmembrane helices are subjected to a further round of epitope prediction in linear-B cells. Using an artificial neural network, the ABCpred server predicts linear B cell epitope areas in an antigen sequence. This server aids in identifying epitope regions that are helpful in choosing candidates for synthetic vaccines, disease diagnosis and allergy studies. ABCpred ( http://crdd.osdd.net/raghava/abcpred/ ) server was used to predict the B-cell epitopes [23]. Epitopes having a value higher than 0.8 were taken into consideration. Furthermore, several types of antibodies, including IgG, IgE, and IgA should be induced by the B-cell epitopes. This antibody-inducing epitope prediction was achieved using IgPred ( http://crdd.osdd.net/raghava/igpred/ ) online tool [24].
Epitope mapping identifies the most promising B-cell and T-cell epitopes for the development of an epitope-based vaccine. T-cell epitopes range in length from 8 to 20 amino acids and can elicit a strong T-cell response. It is critical to determine whether epitopes are immunoprotective before developing a synthetic vaccine peptide [25]. The CTL epitopes are predicted using NetCTL 1.2 servers (http://www.cbs.dtu.dk/services/NetCTL/). The following alleles were chosen to predict the CTL epitopes: HLA-A*0101 (A1), HLA-A*0201 (A2), HLA-A*0301 (A3), HLA-A*2402 (A24), HLA-A*2601 (A26), HLA-B*0702 (B7), HLA-B*0801 (B8), HLA-B*2705 (B27), HLA-B*3901 (B39), HLA-B*4001 (B44), HLA-B*5801 (B58), HLA-B*1501 (B62). The threshold for epitope identification was adjusted at 0.75. A potential CTL epitope is described as one that is non-allergenic, antigenic, and non-toxic. As a result, the predicted CTL epitope’s allergenicity, antigenicity, and toxicity were investigated. The allergenicity of each CTL epitope was determined using Allergen FP v1.0 tool (https://ddg-pharmfac.net/AllergenFP/), while the antigenic nature was predicted using VaxiJen 2.0 web server (http://www. ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html) 26.. Lastly, the toxicity was detected using ToxinPred tool (http://crdd.osdd.net/ raghava/toxinpred/). For further analysis, only the non-allergenic, non-toxic, and antigenic CTL epitopes were selected [25].
HTL (CD4), CTL, regulatory T-cells, and memory T-cells are the four kinds of T-cell lymphocytes that contribute to the T-cell- mediated immune reaction. HTL play an important role in aiding B-cells and CTL in producing antibodies and destroying pathogens, respectively. In addition, they interact with innate immune cells and assist cytotoxic CD8 T cells in expanding and sustaining memory [27]. Considering the importance of HTL in preventing M. tb infection, HTL epitopes were identified using NetMHCII4.0 (http://www.cbs.dtu.dk/services/NetMHCIIpan/) online tool [28]. There are 34 distinct types of Human Leukocyte Antigen (HLA) on this server. The study was only able to examine four alleles, as suggested by Pradhan et al. as the HLA-DR supertype is significant.: HLA-DRB1*0101, HLA-DRB3*0101, HLA- DRB4*0101, and HLA-DRB5*0101 [29]. The threshold for strong interactors and weak interactors was set at 2% and 5% respectively [30].
A crucial cytokine in the regulation of M. tb infection is IFN-γ, a Type II interferon [31]. Presentation by inducing the production of MHC class I and II molecules and boosting CD4 T during infection, CD4 and CD8 T cells both generate this cytokine. Its crucial function is to activate macrophages, allowing them to carry out bactericidal functions. It also improves antigen cell maturation into T-helper type 1 cells. In order to prove that not all MHC class II binders produce the same type of cytokines, the chosen HTL epitopes were exposed to IFN- IL-10 prediction [32]. The IFN-γ inducing epitope was predicted using IFN epitope online tool (http://crdd.osdd.net/ aghava/ifnepitope/index.php) while the IL-10 inducing 34 epitopes were predicted using IL-10Pred (https://webs.iiitd.edu.in/ aghava/il10pred/algo.php) online tool [33]. IFN epitope is an online prediction tool that identifies and creates peptides from protein sequences that can cause CD4+ T lymphocytes to produce IFN-gamma. The IL-10Pred tool can create, locate and map peptides that could activate IL-10.
MHC molecules have a wide range of diversity. More than 225 HLA Class I and 986 HLA Class II allelic variants have been found in humans so far. Furthermore, distinct MHC variations occur at varying rates in various populations, complicating the development of epitope-based vaccines. The identified epitopes trigger a significant immunological response by binding to multiple HLA supertype alleles for maximum population coverage, which is one of the key ideal qualities for multi-epitope vaccine candidates. Finding the best HLA allele collections with the greatest coverage for various populations would be a worthy pursuit in this situation [34]. The shortlisted CTL and HTL epitopes with their respective HLA allele types were submitted to the IEDB population coverage analysis tool (http://tools.iedb.org/population/) [35]. Calculations for MHC class I and MHC class II were done separately. This research focused on the following populations: World, Europe, East Asia, Northeast Asia, South Asia, Southeast Asia, and Southwest Asia.
The next step in this investigation will be to create a multi-epitope vaccine using the shortlisted B-cell, CTL, and HTL epitopes. An adjuvant is a chemical or biological compound with inherent immunostimulatory properties that considerably boosts the host’s immune response in comparison to reactions set off by the epitope alone. In this study, the Heparin-binding hemagglutinin (HBHA) protein of M. avium subsp. paratuberculosis (Accession no. AGV15514.1) was used as an adjuvant. The fact that this protein efficiently induces potent immune responses and functions as a TLR4 activator is the main justification for evaluating it as an adjuvant. In order to achieve prolonged conformation (flexibility), protein folding, and functional domain separation, linkers are necessary. These factors increase the stability of the protein structure [36]. B-cell, CTL, and HTL epitopes were linked with the GGGS linker whereas the adjuvant was linked with the EAAAK linker.
The ProtParam tool (https://web.expasy.org/protparam/) was used to assess the physicochemical properties such as molecular weight, isoelectric pH, aliphatic index, hydropathicity, instability index, GRAVY values and estimated half-life of both multi-epitope vaccine constructs. Further, the nature of the allergenicity of the constructs was identified using Allergen FP tool (https:// ddg-pharmfac.net/AllergenFP/), while the antigenic nature was predicted using VaxiJen 2.0 web server (http://www.ddg-pharmfac. net/vaxijen/VaxiJen/VaxiJen.html) [26].
The proteins were selected on the basis of their importance in the DEG database and their location in the cell’s extracellular area. The localization of previously identified 425 essential proteins was done using PSORTb 3.0 server. According to this tool, only four proteins were found to be extracellular. These four proteins are secreted fibronectin-binding protein C antigen 85-C (Ag85C) (AHJ53223.1), PE-PGRS family protein (AHJ53877.1),invasion protein RipA (AHJ54662.1) and invasion protein RipB (AHJ54663.1). The M. tb Ag85 complex comprises Ag85A, Ag85B, and secreted fibronectin-binding protein C antigen 85-C (Ag85C). Interestingly, this complex is ubiquitous in all Mycobacteria species and has gained a lot of attention due to its significance in M. tb pathogenicity. This Ag85 complex is a major secretory antigen that has been found to be highly immunogenic in both in-vivo and in-vitro analyses. This complex evades the host immunological response by binding to the fibronectin, mycolyltransferas and inhibiting phagosome maturation [37- 39]. Therefore, this complex has numerous applications, such as diagnostics and vaccine candidates. Moreover, Kumar et al. observed that Ag85C had the maximum accuracy of 92% out of all the antigens utilized in ELISA for antibody detection [40]. On the other hand, Ag85 A and B are exploited in the development of vaccine candidates [41]. The following extracellular protein belongs to the PE-PGRS family, which are surface antigens. It is a subgroup of the PE family proteins and has a domain with multiple repeats of Gly-Ala residue. PE-PGRS genes are expressed during the infection, according to Delogu et al. animal model research [42]. Furthermore, PE-PGRS33 is the only PE-PGRS protein that has been thoroughly investigated so far. Lastly, the two invasion proteins RipA and RipB are involved in the invasion of host macrophages. The deletion of RipA makes the pathogen avirulent and increases antibiotic susceptibility. A similar observation was seen upon deletion of RipA in M. smegmatis [43]. Similar to PE-PGRS, the two invasion proteins have limited data available. Therefore, further studies are required to understand the role of these proteins.
Determining the antigenic nature of the protein, which was formerly a time-consuming and expensive process, is now exceedingly simple and inexpensive owing to the bioinformatics revolution of proteomics. VaxiJen is one of the most prevailing computational tools for determining protein antigenicity [43]. In the present analysis, VaxiJen 2.0 was used to predict the antigenic nature of the extracellular proteins. A threshold of 0.4 was set to eliminate non-antigenic proteins. As shown in (Table 1), all proteins were determined to be antigenic since their probability values were more than 0.4.
Immunoinformatics has transformed the role of immunology in the development of diagnostic and vaccine candidates against a broad spectrum of pathogens. Furthermore, it has eased the prediction of B-cell and T-cell epitopes [46]. Linear B-cell epitopes are short antigenic 16-amino-acid sequences that might cause the development of particular antibodies [48]. The ABCpred server was utilized to detect B-cell epitopes in this study. The results revealed that secreted fibronectin-binding protein C antigen 85-C (Ag85C) and invasion protein RipB had 10 B-cell epitopes, whereas invasion protein RipA had 24 B-cell epitopes. As a result, the IgPred server was utilized to eliminate such non-inducing antibody epitopes. According to this tool, there was a significant decrease in epitopes. There were ten epitopes in secreted fibronectin-binding protein C antigen 85-C (Ag85C); however, only three could trigger antibody production. Additionally, invasion proteins RipA and RipB possessed 24 and 10 epitopes, respectively, which were reduced to 5 antibody-inducing epitopes. Detailed information about the antibody-inducing epitopes is given in Table 4.
The adaptive immune system is categorized into humoral immunity and cell-mediated immunity. B-cells are involved in humoral immunity, whereas T-cells are involved in cell-mediated immunity. There are two types of T-cells: cytotoxic T lymphocytes (CD8) and helper T lymphocytes (CD4) [49,50]. The immunological response of T cells works in the following way: Antigens are first detected by antigen-presenting cells (APCs), which then deliver these antigens to T-cells using major histocompatibility complex (MHC) molecules. Epitope mapping is an emerging area of Immunoinformatics that emphasizes on identifying the most promising B-cell and T-cell epitopes for the development of an epitope-based vaccine. T-cell epitopes range in length from 8 to 20 amino acids and can elicit a strong T-cell response. Furthermore, to manage diseases including Rickettsia, Salmonella, Listeria, Mycobacteria, and Chlamydia, a powerful immunogenic response is necessary [51].
In the present study, T-cell epitopes were predicted based on 12 different HLA alleles: HLA-A*0101 (A1), HLA-A*0201 (A2), HLA-A*0301 (A3), HLA-A*2402 (A24), HLA-A*2601 (A26), HLA-B*0702 (B7), HLA-B*0801 (B8), HLA-B*2705 (B27), HLA-B*3901 (B39), HLA-B*4001 (B44), HLA-B*5801 (B58) and HLA-B*1501 (B62). There were 77 CTL epitopes predicted for the secreted fibronectin-binding protein C antigen 85-C (Ag85C) protein58 and 70 for the invasion proteins RipA and RipB, respectively. Refer to (Table. 5)
HTL (CD4), CTL, regulatory T-cells, and memory T-cells are the four kinds of T-cell lymphocytes that contribute to the T-cell- mediated immune reaction. In addition, they interact with innate immune cells and assist cytotoxic CD8 T cells in expanding and sustaining memory [52]. Although the role of CD4 is uncertain, evidence suggests that it is essential for the establishment of an anti-M. tb immune response. Its absence can lead to the pathogen’s reactivation. MHC-II-restricted anti-M. tb responses are studied in depth, and several M. tb antigens targeting CD4 have been discovered so far. The HTL activation requires the presentation of antigens by the MHC-II molecules [50]. Considering the importance of HTL in preventing M. tb infection, the HTL epitopes were predicted using NetMHC-II 4.0 web server. The following alleles were chosen owing to significance in humans: HLA-DRB1*0101, HLA-DRB3*0101, HLA-DRB4*0101, and HLA-DRB5*0101. According to the findings, there were 26 epitopes predicted for the secreted fibronectin-binding protein C antigen 85-C (Ag85C). While 33 HTL epitopes were predicted for the invasion protein RipA, none were predicted for the HLA-DRB3*0101 allele. Similarly, 35 HTL epitopes were predicted for the invasion protein RipB [53].
Cytokines are the signalling molecules produced by different cell types that principally facilitate intercellular communication. A crucial cytokine in the regulation of M. tb infection is IFN-γ, a Type II interferon and CD4 and CD8 T cells both produce this cytokine during infection [54]. The cytokine’s main function is to activate macrophages, allowing them to carry out bactericidal activity. It also improves antigen presentation by inducing the production of MHC class I and II molecules and boosting CD4 T cell maturation into T-helper type 1 cells. In recent studies, patients with lower levels of IFN-γ were found to be more vulnerable to Mycobacterial infections [55,56]. Therefore, to increase the efficacy of the vaccine against M. tb, the HTL epitopes that induce IFN-γ were shortlisted. According to the findings, eight, nine, and ten epitopes from secreted fibronectin-binding protein C antigen 85-C (Ag85C), invasion protein RipA, and invasion protein RipB, respectively, triggering IFN-γ were shortlisted. Interleukin-10 (IL- 10) is another type of cytokine produced by macrophages and T-lymphocytes during M. tb infection. It is also known as a cytokine synthesis inhibitory factor (CSIF) since it prevents T-cells from producing cytokines. It is responsible for macrophage deactivation during M. tb infection, including suppression of IL-12 production, which eventually reduces IFN-γ production. Consequently, the pathogen has a better chance of surviving in this environment [57]. Therefore, it is critical to identify and eliminate the epitopes that activate IL-10 production to control the infection. This prediction was done using the IL-10 Pred server. Only one epitope out of eight IFN-inducing epitopes in secreted fibronectin-binding protein C antigen 85-C was discovered to induce IL-10 production. Similarly, four epitopes in invasion protein RipA were predicted to promote IL-10 production, whereas five epitopes in invasion protein RipB triggered IL-10 production. The list of HTL epitopes that induce IFN-γ and not IL-10 is given in Table 6.
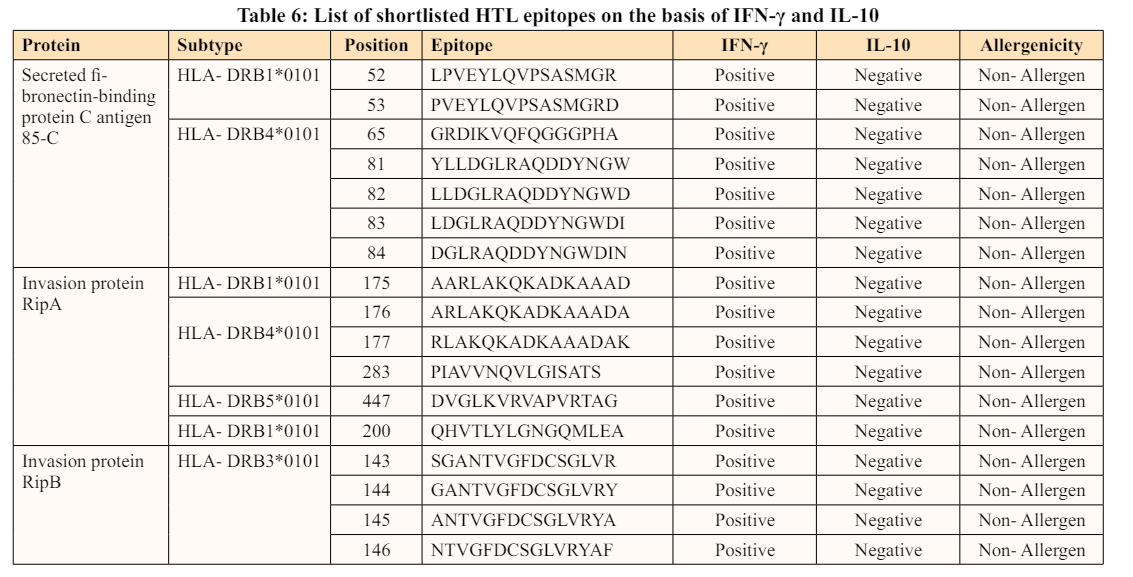
Different populations experience diverse MHC variations at varying rates, complicating the development of epitope- based vaccines. The identified epitopes trigger a significant immunological response by binding to multiple HLA supertype alleles for maximum population coverage, which is one of the ideal qualities for multi-epitope vaccine candidates [58,59].
In the present study, population coverage analysis of the shortlisted CTL and HTL was performed using the IEDB population coverage analysis tool. The following population was considered in this study: East Asia, Europe, Northeast Asia, South Asia, Southeast Asia, Southwest Asia, and World. East Asia covers Japan, Japan Oriental, Korea, South Korea, South Oriental, Mongolia, and Mongolia Oriental. Europe covers Turkey, Italy, Czech Republic, Scotland, France, Georgia, Slovakia, Ireland South, Wales, Norway, Belarus, Slovenia, Germany, Belgium, Spain, Ukraine, Ireland Northern, Netherlands, Denmark, Poland, Finland, Macedonia, Sweden, Croatia, Switzerland, Russia, Bulgaria, Romania, England, Portugal, Serbia, United Kingdom, Austria, and Greece. Northeast Asia covers China and Hong Kong while South Asia covers Sri Lanka, Pakistan, and India. Southeast Asia covers Indonesia, Singapore, Philippines, Borneo, Malaysia, Vietnam, Thailand, and Taiwan whereas Southwest Asia covers Israel, Iran, Oman, Lebanon, Saudi Arabia, Jordan, and UAE.
From Figure 1, it is evident that Europe had the highest population coverage for CTL epitopes of Ag85C (90.80%) and RipA (82.55%), whereas RipB had the lowest population coverage (46.88%). Ag85C had a population coverage of 90.15% worldwide, whereas RipA had a population coverage of 87.28%. On the contrary, RipB had a population coverage of only 30% worldwide, which is extremely low. Further, the population coverage of Ag85C, RipA and RipB in East Asia was found to be 75.90%, 71.49%, and 12.99%, respectively. Ag85C had a population coverage of 50-51%, whereas RipA had a 40-41% population coverage in Northeast and South Asia. In comparison, RipB had a population coverage of 6.42% and 17.93% in Northeast and South Asia. In Southeast and Southwest Asia, the population coverage of Ag85C was 56.46 % and 56.80%, respectively. Similarly, the population coverage of RipA was 56.97% and 46.91% in Southeast and Southwest Asia, respectively. On the other hand, the population coverage of RipB was extremely low for all the populations taken into consideration. This indicates that the predicted CTL epitopes for the selected protein do not cover these populations. Hence, it cannot be taken further for vaccine construction.
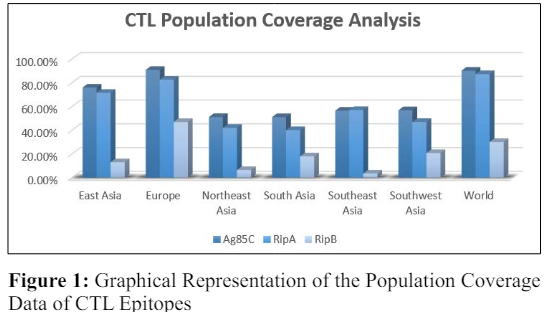
Figure 2 shows that the HTL epitopes of Ag85C (88.90%) and RipA (89.45%) covered the maximum population of the world, whereas RipB covered only 24.10% population of the world. The population coverage in Europe for Ag85C, RipA and RipB was 85.60%, 80.67%, and 50.34%, respectively. Ag85C covered 72.80% of the East Asian population, whereas RipA covered 78.36%, and RipB covered 20.45% of the East Asian population. Ag85C had 62.45% and 55.19% population coverage in Northeast and Southeast Asia, respectively, whereas RipA had 54.85% and 57.21%. RipB, on the other hand, barely covered 10.04 percent and 12.80 percent of the population in Northeast and Southeast Asia, respectively. In South and Southwest Asia, the population coverage of Ag85C was 52.76% and 54.90%, respectively. Similarly, the population coverage of RipA was 51.72% and 56.09% in South and Southwest Asia, respectively. Thus, it was observed that the population coverage of RipB CTL and HTL epitopes covered a small proportion of the population, making it a less effective vaccine candidate when used by such a population. Therefore,further research should be conducted to determine whether it covers the rest of the population listed on the server in order to be used as a viable vaccination candidate.
The next step in this analysis was to construct the multi-epitope vaccine using the shortlisted B-cell, CTL, and HTL epitopes. In this study, the Heparin-binding hemagglutinin (HBHA) protein of M. avium subsp. paratuberculosis (Accession no. AGV15514.1) was used as an adjuvant. The fact that this protein efficiently induces potent immune responses and functions as a TLR4 activator is the main justification for evaluating it as an adjuvant [60]. Linkers are essential for achieving prolonged conformation (flexibility), protein folding, and functional domain separation, hence making the protein structure more stable. The adjuvant was joined with the EAAAK linker, whereas B-cell, CTL, and HTL epitopes were joined with the GGGS linker. Construct 1 refers to the vaccine developed from epitopes (Figure 3), whereas Construct 2 refers to the vaccine constructed from RipA epitopes (Figure 4).

ExPASy ProtParam Server was used to evaluate the physicochemical parameters, including molecular weight, theoretical isoelectric point (pI), aliphatic index, instability index, and GRAVY value of the two vaccine constructs. Construct 1 and 2 have a molecular weight of 56.09 and 53.83 kDa, respectively, which is less than 100 kDa, indicating that they would be quick and easy to purify. The isoelectric point (pI) is where the net charge on amino acid is zero as it produces an equal amount of positive and negative ions. A high pI indicates basic amino acid, whereas acidic amino acid has a low pI value. Construct 1 has a pI of 4.72, while Construct 2 has a pI of 9.27. This indicates that Construct 1 is acidic in nature, whereas Construct 2 is basic.
Another physiochemical property is the instability index, which approximates the stability of a protein. If the instability index of a protein is more than 40, it is considered unstable. The instability index of Construct 1 was predicted to be 47.59, which is greater than 40, indicating that this construct is unstable. On the contrary, the instability index of Construct 2 was estimated to be 35.77, indicating that it is stable. The aliphatic index is used to define how much volume of a protein is taken up by aliphatic side chains (valine, leucine, isoleucine, and alanine). It is a measurement of the thermostability of a protein. A high aliphatic index protein is more thermally stable. The aliphatic index of both constructs was in the range of 60-70, suggesting that they are thermally stable. Lastly, the grand average of hydropathicity (GRAVY) value is used to calculate the Hydropathicity average. A protein with a positive GRAVY value is said to be hydrophobic and non-polar, while one with a negative GRAVY value is said to be hydrophilic, polar, and better at interacting with water. The GRAVY value of Constructs 1 and 2 was found to be -0.58 and -0.425, respectively. This suggests that both proteins are hydrophilic in nature. Further, the allergenicity and antigenicity of both the constructs were evaluated using AllergenFP and VaxiJen server. Both constructs were shown to be non-allergenic and antigenic, implying that they can elicit a powerful immunogenic response without causing allergy responses in the host. To summarise, the results indicate that the construct 2 is physiochemically suitable for vaccine production, whereas construct 1 requires certain modifications before it can be considered a vaccine candidate. The details of the physiochemical properties, allergenicity and antigenicity nature of the two constructs is given in Table 7.
The emergence of MDR strains has aggravated the problem of tuberculosis making it a major public health concern worldwide. The computational approaches presented in this paper have the potential to provide better understanding M. tb vaccine antigens and vaccine candidates that cannot be obtained through pre- clinical, in-vitro, or animal studies. An immuno-informatics tool was used in this study to define a tuberculosis novel multi-epitope subunit vaccine that is highly immunogenic and has the properties of a carrier vaccine and for which the whole proteome of M. tb CCDC5180 was analyzed. Using the subtractive proteomics method, four extracellular proteins such as RipA, RipB, PE-PGRS and Ag85C protein were discovered and used in the vaccine candidate analysis. Each of these proteins was examined for the presence of signaling peptides and antigenicity. Multiple B-cell and T-cell epitopes were analyzed using epitope prediction to improve the vaccine’s immunogenicity. Similarly, HTL epitopes that trigger IFN-γ but not IL-10 were selected. In addition, the CTL and HTL epitopes of the two proteins Ag85C and RipA were chosen since they covered the maximum population. Finally, multi-epitope vaccines were constructed using the shortlisted epitopes. The vaccine’s antigenicity, allergenicity, solubility and, physiochemical properties were all found to be very satisfactory for Rip A protein. However, in-vitro and in-vivo experiment validation is required to confirm the construct’s potency. The long-term goal of the study is to predict the tertiary structure of the identified vaccine targets. Another aim could be to predict more HTL epitopes based on the remaining HLA alleles available on the NetMHCII server. The vaccine can then be docked with human TLR receptors to validate the RipA vaccine design using molecular dynamics simulation tests.
1. Weiner J, S H E Kaufmann (2014) Recent advances towards tuberculosis control: Vaccines and biomarkers. J. Intern. Med 275: 467-480.
2. J Montoya, Juan Antonio Solon, Soledad Rosanna C Cunanan, Luz Acosta, Anne Bollaerts, et al. (2013) A randomized, controlled dose-finding Phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J. Clin. Immunol 33: 1360-1375.
3. L M Marques Neto, A Kipnis, A P Junqueira-Kipnis (2017) Role of metallic nanoparticles in vaccinology: Implications for infectious disease vaccine development. Front. Immunol 8: 239.
4. K Floyd et al. Global tuberculosis report, 1-277, 2018 https:// www.aidsdatahub.org/sites/default/files/resource/who-global- tb-report-2018.pdf
5. K Zaman (2010) Tuberculosis A global health problem, J Heal Popul Nutr 28: 111-113.
6. P F McDermott, R D Walkerand D G White (2003) Antimicrobials Modes of action and mechanisms of resistance, Int J Toxicol 22: 135-143.
7. M A Forrellad, Laura I Klepp, Andrea Gioffré, Julia Sabio y García, Hector R Morbidoni, María de la Paz Santangelo, et al. (2013) Virulence factors of the mycobacterium tuberculosis complex Virulence 4: 3-66.
8. M Gengenbacher, S H E Kaufmann (2012) Mycobacterium tuberculosis Success through dormancy FEMS Microbiol Rev 36: 514-532.
9. S M Gygli, S Borrell, A Trauner, S Gagneux (2017) Antimicrobial resistance in Mycobacterium tuberculosis Mechanistic and evolutionary perspectives FEMS Microbiol. Rev 41:354-373.
10. Y Zhang, Chen Chen, Jie Liu, Haijun Deng, Aizhen Pan, Lishui Zhang, et al. (2011) Complete genome sequences of Mycobacterium tuberculosis Strains CCDC5079 and CCDC5080, which belong to the Beijing family, J Bacteriol 193: 5591-5592.
11. B N Bowman, Paul R McAdam, Sandro Vivona, Jin X Zhang, Tiffany Luong, Richard K Belew, et al. (2011) Improving reverse vaccinology with a machine learning approach Vaccine 29: 8156-8164.
12. K L Seib, X Zhao, R Rappuoli (2012) Developing vaccines in the era of genomics A decade of reverse vaccinology Clin Microbiol Infect. 18 SUPPL 5 :109-116.
13. J Adu-Bobie, B Capecchi, D Serruto, R Rappuoli, M Pizza (2003) Two years into reverse vaccinology Vaccine 21: 605-610.
14. A Ali, Shabir Ahmad, Abdul Wadood, Ashfaq U Rehman, Hafsa Zahid, et al. (2020) Correction Modeling novel putative drugs and vaccine candidates against tick-borne pathogens A subtractive proteomics approach doi: 10.3390/vetsci7040158.
15. V Solanki, M Tiwari, V Tiwari (2019) Prioritization of potential vaccine targets using comparative proteomics and designing of the chimeric multi-epitope vaccine against Pseudomonas aeruginosa Sci. Rep 9: 1-19.
16. H A Dar, Tahreem Zaheer, Muhammad Shehroz, Nimat Ullah, Kanwal Naz, Syed Aun Muhammad et al. (2019) Immunoinformaticss-aided design and evaluation of a potential multi-epitope vaccine against klebsiella pneumonia Vaccines 7: 1-17.
17. V Solanki, V Tiwar (2018) Subtractive proteomics to identify novel drug targets and reverse vaccinology for the development of chimeric vaccine against Acinetobacter baumannii Sci. Rep 8: 1-19.
18. I A Doytchinova, D R Flower (2007) VaxiJen A server for prediction of protective antigens tumour antigens and subunit vaccines BMC Bioinformatics 8: 1-7.
19. E R Green, J Mecsas (2016) Bacterial secretion systems an overview Virulence Mech. Bact. Pathog.4: 213-239.
20. K Frank, M J Sippl (2008) High-performance signal peptide prediction based on sequence alignment techniques Bioinformatics 24: 2172-2176.
21. A Krogh, B Larsson, G Von Heijne, E L L Sonnhammer (2001) Predicting transmembrane protein topology with a hidden Markov model Application to complete genomes J Mol. Biol 305: 567-580.
22. B Korber, M LaBute, K Yusim (2006) Immunoinformatics comes of age PLoS Comput. Biol 2: 0484-0492.
23. S Mukhopadhyay, S Nair, S Ghosh (2012) Pathogenesis in tuberculosis Transcriptomic approaches to unraveling virulence mechanisms and finding new drug targets FEMS Microbiol. Rev 36: 463-485.
24. S Gupta, H R Ansari, A Gautam, and G P S Raghava (2013) Identification of B-cell epitopes in an antigen for inducing specific class of antibodies Biol. Direct 8: 1.
25. M V Larsen, C Lundegaard, K Lamberth, S Buus, O Lund, and M. Nielsen (2007) Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction BMC Bioinformatics 8: 1-12.
26. I Dimitrov, L Naneva, I Doytchinova, I Bangov (2014) AllergenFP Allergenicity prediction by descriptor fingerprints Bioinformatics 30: 846-851.
27. D S Rosa, S P Ribeiro, E Cunha-Neto (2010) CD4+ T cell epitope discovery and rational vaccine design Arch. Immunol. Ther. Exp. (Warsz) 58: 121-130.
28. B Reynisson Carolina Barra, Saghar Kaabinejadian, William
H. Hildebrand, Bjoern Peters, and Morten Nielsen et al. (2020) Improved Prediction of MHC II Antigen Presentation through Integration and Motif Deconvolution of Mass Spectrometry MHC Eluted Ligand Data J Proteome Res. 19: 2304-2315.
29. D Pradhan, M Yadav, R Verma, N S Khan, L Jena, A K Jain (2017) Discovery of T-cell Driven Subunit Vaccines from Zika Virus Genome an Immunoinformatics Approach Interdiscip. Sci. – Comput. Life Sci 9: 468-477.
30. V Priyadarshini, D Pradhan, M Munikumar, S Swargam, A Umamaheswari, D Rajasekhar (2014) Genome-based approaches to develop epitope-driven subunit vaccines against pathogens of infective endocarditis J Biomol Struct. Dyn 32: 876-889.
31. Y V N Cavalcanti, M C A Brelaz, J K D A L Neves, J C Ferraz, and V R A Pereira (2012) Role of TNF-alpha, IFN-gamma,IL-10 in the development of pulmonary tuberculosis Pulm. Med 2012: 2012.
32. N Khairkhah, M R Aghasadeghi, A Namvar, A. Bolhassani(2020)Design of novel multiepitope constructs- based peptide vaccine against the structural S N M proteins of human COVID-19 using immunoinformatics analysis PLoS One 15: 1-28.
33. S K Dhanda, P Vir, G P S Raghava (2013) Designing of interferon-gamma inducing MHC class-II binders Biol. Direct 8: 1-15.
34. J Davila, L A McNamara, Z Yang (2012) Comparison of the predicted population coverage of tuberculosis vaccine candidates Ag85b-ESAT-6, Ag85B-TB10.4, and Mtb72f via a bioinformatics approach PLoS One 7: 1-11.
35. H H Bui, J Sidney, K Dinh, S Southwood, M J Newman,A. Sette (2006) Predicting population coverage of T-cell epitope- based diagnostics and vaccines BMC Bioinformatics 7: 1-5.
36. D J Park, “PCR Protocols Edited by.” https://catalogue.nla.gov.au/Record/5039030
37. H G Wiker, M Harboe (1992) The antigen 85 complex A major secretion product of Mycobacterium tuberculosis Microbiol. Rev 56: 648-661.
38. K Huygen (2014) The immunodominant T-cell epitopes of the mycolyl-transferases of the antigen 85 complex of M. tuberculosis Front. Immunol. 5 JUL 1-11.
39. C J Kuo, H Bell, C L Hsieh, C P Ptak, Y F Chang (2012) Novel mycobacteria antigen 85 complex binding motif on fibronectin J Biol. Chem. 287: 1892-1902.
40. G Kumar, P K Dagur, M Singh, V S Yadav, R Dayal, et at. (2008) Diagnostic potential of Ag85C in comparison to various secretory antigens for childhood tuberculosis Scand J Immunol 68: 177-183.
41. M Karbalaei Zadeh Babaki, S Soleimanpour, S A Rezaee (2017) Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene Biology, immune-pathogenicity, applications in diagnosis, and vaccine design Microb. Pathog 112: 20-29.
42. G Delogu, M J Brennan (2001) Comparative immune response to PE and PE_PGRS antigens of Mycobacterium tuberculosis Infect. Immun 69: 5606-5611.
43. D Böth, G Schneider, R Schnell (2011) Peptidoglycan remodeling in mycobacterium tuberculosis: Comparison of structures and catalytic activities of RipA and RipB J Mol. Biol. 413: 247-260.
44. D L Leyton, A E Rossiter,I R Henderson (2012) From self- sufficiency to dependence Mechanisms and factors important for autotransporter biogenesis Nat. Rev. Microbiol 10: 213- 225.
45. T A Ahmad, A E Eweida, S A Sheweita (2016) B-cell epitope mapping for the design of vaccines and effective diagnostics Trials Vaccinol 5: 71-83.
46. M Bhasin, G P SRaghava (2005) Pcleavage an SVM based method for prediction of constitutive proteasome and immunoproteasome cleavage sites in antigenic sequences Nucleic Acids Res. 33: 202-207.
47. D Kladnik (2012) Slovenian geography and geographical names Geogr. Vestn. 84: 237-249.
48. U Kulkarni-Kale, S Bhosle, A S Kolaskar (2005) CEP: A conformational epitope prediction server. Nucleic Acids Res 33: 168-171.
49. A Patronov, I Doytchinova (2013) T-cell epitope vaccine design by immunoinformatics.Patronov A & Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Open Biology 3: 120139.
50. N Tomar, R K De (2010) Immunoinformatics An integrated scenario Immunology 131: 153-168.
51. T A Ahmad, A E Eweida, L H El-Sayed (2016) T-cell epitope mapping for the design of powerful vaccines Vaccine Reports 6: 13-22.
52. M Valentino, J Frelinger (2016) genomic era: application to Francisella tularensis 45: 218–228.
53. J Wei, J L Dahl, J W Moulder, E A Roberts, P O’Gaora, D B Young, R L Friedman et al. (2000) Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages J Bacteriol 182: 377-384.
54. JA LFlynn, M M Goldstein, J Chan, K J Triebold, K Pfeffer, C J Lowenstein, R Schreiber, T W Mak et al (1995) Tumor necrosis factor-α is required in the protective immune response against mycobacterium tuberculosis in mice Immunity 2: 561–572.
55. T A Khan, H Mazhar, S Saleha, H N Tipu, N Muhammad, M N Abbas (2016) “Interferon-Gamma Improves Macrophages Function against M. tuberculosis in Multidrug-Resistant Tuberculosis Patients,” Chemother. Res. Pract 1-6.
56. J L Flynn, J Chan (2010) Mmunology of 1-39.
57. P S Redford, PJ Murray, A OGarra (2011) The role of IL-10 in immune regulation during M. tuberculosis infection Mucosal Immunol 4: 261-270.
58. S J Lee, Sung Jae Shin, Moon Hee Lee, Min-Goo Lee, Tae Heung Kang, Won Sun Park, Byoung Yul Soh, et al. (2014) A potential protein adjuvant derived from Mycobacterium tuberculosis Rv0652 enhances dendritic cells-based tumor immunotherapy PLoS One 9: 1-11.
59. AS Mustafa, YASkeiky, R Al-Attiyah, M RAlderson, RG Hewinson, et al. (2006) Immunogenicity of Mycobacterium tuberculosis antigens in Mycobacterium bovis BCG- vaccinated and M bovis-infected cattleInfect. Immun 74: 4566-4572.
60. G L Morefield, LDHawkins, STIshizaka, TLKissner, and RGUlrich (2007) Synthetic toll-like receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome Clin. Vaccine Immunol 14: 1499-1504.
View PDF