Author(s): <p>Ghfran Naif Rahman and Nief Rahman Ahmad*</p>
A fast, greener, simple, accurate, precise, rapid, economical and high sensitive ultraviolet spectrophotometric method has been developed for the determination of Thiamine Chloride Hydrochloride (Vitamin B1) in pharmaceutical preparations and environmental wastewater samples, which shows maximum absorbance at 235 nm in distilled water. Beer’s law was obeyed in the range of 2.5-30μg/ ml, with molar absorptivity of 1.028x104 L.mol-1.cm-1, relative standard deviation of the method was less than 1.9%, and accuracy (average recovery %) was 100 ± 0. 8. No interference was observed from common excipients and additives often accompany with Thiamine Chloride Hydrochloride in pharmaceutical preparations. The method was successfully applied to the determination of Thiamine Chloride Hydrochloride in some pharmaceutical formulations (tablets) and industrial wastewater samples. The proposed method was validated by sensitivity and precision which proves suitability for the routine analysis of Thiamine Chloride Hydrochloride in true samples.
Thiamine chloride Hydrochloride or vitamin B1 (sulfur containing vitamin) is the first vitamin of the water soluble B complex group category of vitamins. The chemical name is 3-(4-Amino-2- methylpyrimidin-5-ylmethyl)-5-(2-hydroxyethyl)-4- methylthiazolium chloride hydrochloride.
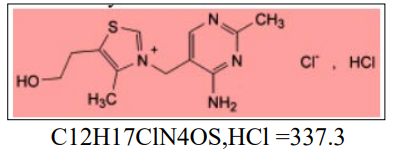
Figure 1: Chemical Structure of Thiamine Hydrochlorid
Thiamine hydrochloride deficiency develops when the dietary intake is inadequate; severe deficiency leads to the development of a syndrome known as beriberi [1, 2]. There are several methods for determination of Thiamine hydrochloride have been described in the literature, these include Only a few HPLC, Spectrofluorimetric, Amperometric, Chemo metric, Capillary electrophoreses, Ion pair Liquid Chromatography and Spectrophotometric [3-13].
In the view of the need in the industry for routine analysis of thiamine hydrochloride, attempts are being made to develop simple and accurate instrumental methods for quantitative estimation of thiamine hydrochloride. This paper reports a simple, accurate, and precise spectrophotometric method for the determination of thiamine hydrochloride in pure form, pharmaceutical formulations, and application to content uniformity testing, The ultraviolet spectrophotometric method is the instrumental method of choice commonly used in industrial laboratories because of their simplicity, selectivity, as of our, for this reason an attempt has been made to developed new UV method for determination of Thiamine Chloride Hydrochloride in pharmaceutical preparations and environmental wastewater samples with high absorption value at 235 nm, result in increasing sensitivity, good accuracy, simplicity, precision and economy [14-21].
Spectro-scan 50 UV-visible (double beam) spectrophotometer with 1.0 cm quartz cells was used for absorption measurements
All chemical used were of analytical or pharmaceutical grade and Thiamine Chloride Hydrochloride standard material was provided from the state company for pharmaceutical industries (NDI) Mosul- Iraq.
Thiamine Chloride Hydrochloride Standard Solution 10ppm This solution was prepared by dissolving 10 mg of Thiamine Chloride Hydrochloride in 1000 ml of distilled water in calibrated flask.
The standard solution of Thiamine Chloride Hydrochloride (10µg/ ml) was scanned in the range of 220300nm which show maxima located at 235 nm Figure 2. Therefore, this wavelength was used for the construction of calibration curve.
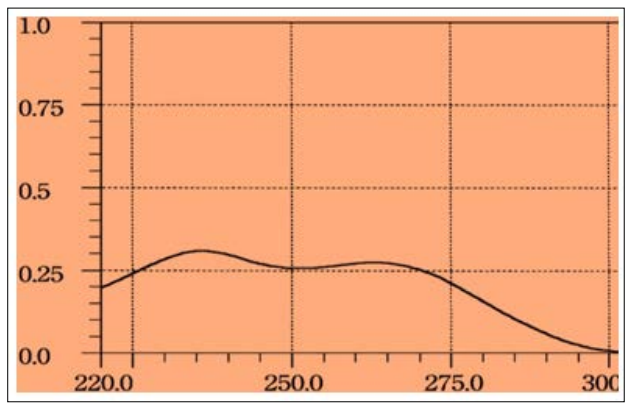
Figure 2: Absorption spectra of 10µg/ml Thiamine Chloride Hydrochloride Against Distilled Water
From the absorption maxima, calibration curve was prepared in the concentration range of 2.5-30 µg/ml. The absorbance was measured at 235 nm against distilled water as a blank. The concentration of the sample solution can be determined by using the calibration curve.
For the determination of Thiamine Chloride Hydrochloride in tablet preparations ,and to minimize a possible variation in the composition of the tablets, the composition of the [Tablets of Thiamine Chloride Hydrochloride 10 mg/tab] were provided from the state company of drug industries and medical (NDI) Nineveh- Iraq .The content of ten tablets of the brand , were weighed and grounded to fine powder, then the powder equivalent to 100 mg of Thiamine Chloride Hydrochloride was stirred well with about 90 ml of distilled water for 20 minutes and the volume was made to 100mL with distilled water ,filtered through what man No. 41 filter paper and 10 ml of this solution was diluted to 100 ml by distilled water to get 10µg/ml solution and treated as described above for recommended procedure and the concentration was calculated by using the calibration curve of this method.
To demonstrate the practical applicability of the proposed method, real water samples were analyzed by this method. Industrial waste water from the state company for drug industries and medical appliances Mosul-Iraq, were fortified with the concentrations in the range of 5, 20, 30 µg/ml of Thiamine Chloride Hydrochloride. The fortified water samples were analyzed as described above for recommended procedure and the concentration was calculated by using the calibration curve of this method.
UV- Visible spectrophotometry is still considered to be a convenient and low cost method for the estimation of pharmaceuticals [22-27].
This method used for the determination of Thiamine Chloride Hydrochloride in pharmaceutical preparations and environmental wastewater samples was found to be high sensitive, simple, accurate ,and reproducible. Beer s law was obeyed in the concentration range of 2.5 - 30 µg/ml. Figure 3 with correlation coefficient of 0.9985, intercept of 0.0013 and slope of 0.0305. The conditional molar absorptivity was found to be 1.028 x104 l/mol.cm. and the limit of detection and limit of quantification were evaluated as LOD = Intercept /Slopex10 and LOQ = 3.3LOD. The limit of detection was 4.26x103 µg/ml and the limit of quantification 14x10-3µg/ml as the lowest standard concentration which could be determine with acceptable accuracy [28, 29].
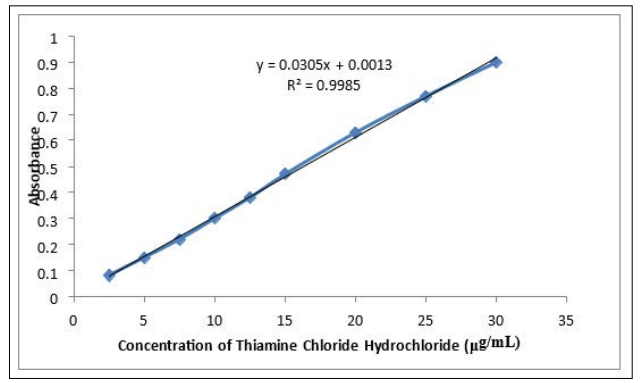
Figure 3: Calibration Graph of Thiamine Chloride Hydrochloride
The accuracy and precision of the method, a pure drug solution was analyzed at three different concentrations, each determination being repeated six times. The relative error (%) and relative standard deviation values are summarized in table 1. From table 1 the values of standard deviation were satisfactory and the recovery studies were (average recovery %) was 100 ± 0. 8. The RSD% value is less than 1.9 indicative of accuracy of the method. the result is compiled in Table 1.
|
Thiamine Chloride Hydrochloride taken µg/ml |
Er (%)a |
RSD(%) |
|
5 |
0.8 |
1.8 |
|
20 |
0.6 |
1.6 |
|
30 |
0.5 |
1.4 |
a: Mean of Six Determinations
In order to assess the possible applications of the proposed method, the effect of substance that often accompany with Thiamine Chloride Hydrochloride in (Tablets) were studied by adding different number of substances to 20 µg of Thiamine Chloride Hydrochloride. An attractive feather of the method is its relative freedom from interference by the usual diluents and excipients in amounts for in excess of their normal occurrence in pharmaceutical preparations. The results are given in table 2.
|
Interfering substances |
Amount added/mg of interfering |
Amount of drug found*(µg) |
RSD % |
|
Lactose |
10 |
20.06 |
1.71 |
|
Microcrystalline cellulose |
20 |
20.05 |
1.64 |
|
Corn starch |
30 |
20.08 |
1.78 |
|
Povidene |
30 |
20.05 |
1.79 |
|
Magnesium stearate |
40 |
20.07 |
1.91 |
|
Hydroxyl propyl methyl cellulose
|
40 |
20.08 |
1.93 |
|
Poly ethylene glycol |
20 |
20.01 |
0.91 |
|
Titanium dioxide |
10 |
20.05 |
0.88 |
Average of six determinations
The proposed method was satisfactorily applied to the determination of Thiamine Chloride Hydrochloride in its pharmaceutical preparations tablets and wastewater samples, the results of the assay of the pharmaceutical preparations revels that there is close agreement between the results obtained by the proposed method and the label claim Table 3, and the results of water samples Table 4 show that the recovery values obtained were closed to 100%.
|
Pharmaceutical formulations |
Label amount (mg) |
Found by proposed method *mg |
Recovery% |
|
Tablets |
100mg/tab |
100.02 |
100.02 |
Table 4: Determination of Thiamine Chloride Hydrochloride in Wastewater Samples
|
Wastewater samples |
Added µg/ml |
Found* (µg/ ml) |
Recovery %(n=10) |
|
Industrial wastewater |
5 |
5.02 |
100.4 |
|
20 |
20.09 |
100.45 |
|
|
30 |
30.08 |
100.26 |
* Mean Value of Ten Determinations.
Application of the Proposed Method to Content Uniformity Content uniformity or the Uniformity of dosage unit was defined as the degree of uniformity in the amount of active substance among dosage units [30-35]. The risk assessment strategy underlying content uniformity testing is the assumption that some pre-specified limits exist where safety and efficacy outcomes may change if content uniformity fails. The proposed method proved to be suitable for the content uniformity test, where a great number of assays on individual tablets are required. Data presented in table 5, indicate that the proposed method cans accurately and precisely quantitative promethazine hydrochloride in its commercially available tablets. The mean percentage (with RSD) of the labeled claim found in ten tablets was 100.61 (0.98% which fall within the content uniformity limits specified by the Japanese Pharmacopoeia [31].
|
Parameter |
% of the label claim |
|
Tablet No.1 |
100.6 |
|
Tablet No.2 |
100.7 |
|
Tablet No.3 |
100.9 |
|
Tablet No.4 |
100.9 |
|
Tablet No.5 |
99.8 |
|
Tablet No.6 |
100.9 |
|
Tablet No.7 |
100 |
|
Tablet No.8 |
100.8 |
|
Tablet No.9 |
100.4 |
|
Tablet N0.10 |
101.1 |
|
Mean(X) |
100.61 |
|
%RSD |
0.98 |
|
Max. allowed unit value [33] |
±15% |
Conclusion
The developed method is found to be highly sensitive, accurate, simple, precise and economical, and can be used for routine quality control analysis of Thiamine Chloride Hydrochloride in pure form, bulk, pharmaceutical formulations and environmental wastewater samples.
The author [Nief Rahman Ahmad] wishes to express gratitude to his former company [the state company of drug industries and medical appliance (NDI) Nineveh-Iraq] for providing gift samples of Thiamine Chloride Hydrochloride standard materials and tablets100 mg/tab).
