Author(s): Piyu Parth Naik
Background: Melasma is a common acquired hyperpigmentation of sun-exposed areas. It is highly treatment resistant and with high chances of recurrence. Treatment of melasma using oral tranexamic acid (TA) with antioxidants and glycolic acid is a novel concept.
Objective: Aim of the study was to assess efficacy and safety of novel combination therapy for the treatment of resistant melasma.
Method: It was a prospective interventional trial conducted on a total of 10 patients with melasma, who did not respond to topical skin lightening therapy. The severity and extent of pigmentation was assessed by modified melasma area severity index (mMASI). Patients were then prescribed oral TA with high dose of vitamin C, Iron & folic acid along with local glycolic acid. The response to treatment was assessed by mMASI and clinical photographs at 8th week and after 3 months on completion.
Result: Significant difference between mMASI was observed among the study population. 60% patients showed 100% improvement and 40% showed 75% improvement from the baseline mMASI at 8 weeks after commencing treatment and 100% patients showed 100% improvement on 3 months completion.
Conclusion: Based on the study results, oral TA in combination with antioxidants and glycolic acid can be used as a potential new, effective, and safe treatment for resistant melasma.
Melasma, formerly known as chloasma, is an acquired pigmentary condition, occurring most commonly on the face. This disorder, which is more prevalent in females and darker skin types, is predominantly attributed to ultraviolet (UV) exposure and hormonal influences.1 Melasma presents as symmetrically distributed hyperpigmented macules, which can be confluent, reticulated, or punctate. Histologically, melasma can display increased epidermal and/or dermal pigmentation, enlarged melanocytes, increased melanosomes, solar elastosis, dermal blood vessels, and, occasionally, perivascular lympho-histiocytic infiltrates [1].
Traditionally melasma is treated with topical skin lightening agents, hydroquinone, chemical peels, dermabrasions, laser treatment and photoprotection. However, the treatment of melasma poses challenge due to the high rate of recurrence and treatment resistance.
Over the years use of tranexamic acid (TA) in topical, oral, or intradermal form has emerged as a novel concept for the treatment of melasma. TA is an antifibrinolytic, which is used to improve blood clotting. Some researchers found that TA has hypopigmentory effect on melasma lesions and also prevents UV-induced pigmentation.2 Intracellular release of arachidonic acid, a precursor of prostanoid and the level of alpha-melanocytestimulating hormone increase as the result of plasmin activity. These two substances can activate melanin synthesis. Therefore, the anti-plasmin activity of TA is thought as the main mechanism of hypopigmentory effect of this agent [2].
The aim of the study was to determine safety and efficacy of oral TA in melasma.
The study was conducted over the period of one year from 01 March 2019 till 01 March 2020 at medical centers in Anand and Surat, India. The sample size was 10
All the patients with melasma who have not responded to topical skin lightening therapy
• Pregnant and lactating women.
• Women taking hormonal contraceptives.
• Subjects with history of thrombotic disorders, seizures,
hypersensitivity reactions, visual disturbances and dizziness.
Patients who met fixed criteria were included in the study. After explaining about the study, informed consent was taken from all the participants. Detailed history regarding duration and previous treatments taken for melasma, any other illnesses, family history and occupation were taken from the participants. Pregnancy was excluded by history and beta-hcg test in female participants. The lesions were examined under woods lamp and dermoscope.
Modified melasma area and severity index (mMASI) scoring system was used to assess the severity of melasma and to compare the response in each patient with their baseline score to find the improvement [3].
Participants were prescribed oral TA (250 mg twice a day for 3 months), high dose of vitamin C and standard doses of Iron, folic acid. All the participants were given SPF50 sunscreen for local application for the duration of study participation along with glycolic acid in cream form. Clinical photographs were taken and mMASI score assessment were done at the beginning, at 8th week; and used for final response assessment. Adverse events if any, were noted at each visit. Study subjects were examined and mMASI score was assessed at 3-month follow-up visit for any further adverse events or recurrence.
All the 10 subjects belonged to age group 29 to 45 years. Participants consisted of eight females and two males. All subjects were of skin type III; except one subject, who was of skin type IV. Seven subjects had centro-facial and three patients had malar distribution. Out of ten subjects, one had dermal, three had epidermal and six had mixed melasma.
| Variables | Number of patients | |
|---|---|---|
| Age group (Years) | 25-30 | 2 |
| 31-35 | 2 | |
| 36-40 | 3 | |
| 41-45 | 3 | |
| Gender | Female | 8 |
| Male | 2 | |
| Pattern of distribution | Centro-facial | 7 |
| Malar | 3 | |
| Mandibular | 0 | |
| Type | Epidermal | 3 |
| Dermal | 1 | |
| Mixed | 6 | |
| Subjects | Baseline | 8th week | % improvement at 8th week | 3-month follow-up | % improvement at 3-month follow-up |
|---|---|---|---|---|---|
| 1 | 18 | 0 | 75 | 0 | 100 |
| 2 | 22 | 0 | 100 | 0 | 100 |
| 3 | 10 | 0 | 100 | 0 | 100 |
| 4 | 08 | 2 | 75 | 0 | 100 |
| 5 | 10 | 0 | 100 | 0 | 100 |
| 6 | 20 | 0 | 100 | 0 | 100 |
| 7 | 18 | 4 | 75 | 0 | 100 |
| 8 | 14 | 0 | 100 | 0 | 100 |
| 9 | 08 | 0 | 75 | 0 | 100 |
| 10 | 18 | 0 | 100 | 0 | 100 |
| Mean mMASI | Baseline | 8th week | % Improvement | 3-month follow-up | % Improvement |
|---|---|---|---|---|---|
| TA | 14.6 | 2.95 | 85.89 | 0 | 100 |
With combination treatment, 60% subjects showed 100% improvement and 40% subjects showed 75% improvement in mMASI score at 8th week. Average mMASI at baseline was 14.6 which improved to 2.95 at 8th week showing 85.89% improvement. (Figure 1-2)
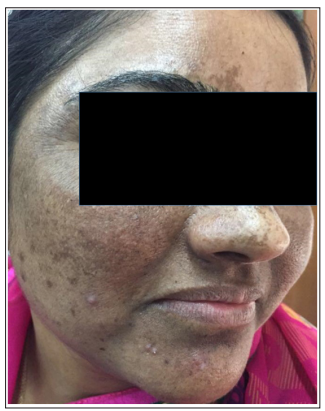
Figure 1: Right lateral oblique image of melasma patient “A” in pre-treatment period
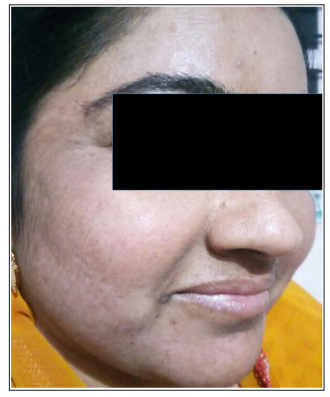
Figure 2: Right lateral oblique image of patient “A” on completion of treatment
At three months follow up all the subjects showed 100% improvement in mMASI score along with 100% patient satisfaction score.
There were no cases of recurrence at 3 months follow up. (Figure 3-4)
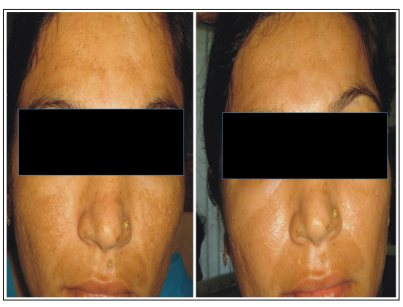
Figure 3,4: Frontal images of patient “B” prior to (on left) and on completion (on right) of treatment
Tranexamic acid is believed to act in melasma by preventing the activation of melanocytes from UV light, hormones, and injured keratinocyte through the inhibition of plasminogen activator system present in epidermal basal cells and keratinocytes. It also reduces melanocyte tyrosinase activity by suppressing the production of prostaglandins and has an additional effect on the dermal blood vessels as it decreases the angiogenesis via inhibition of vascular endothelium growth factor[4].
Various clinical trials have been conducted with oral, topical and intradermal TA to evaluate its efficacy and safety for the treatment of melasma.
Khurana et al. conducted a prospective, randomized, open-label study with a sample size of 64 [5].Thirty-two patients were administered localized microinjections (4mg/mL) of TA monthly in one arm, while in the other arm, 32 were given oral TA 250mg twice a day. Improvement in MASI score in the oral group was 57.5% as compared to 43.5% in the intralesional group. The study noted that, the dose of TA in microinjections and the frequency of injections could have been increased.
Lee et al. conducted a prospective open pilot study of 12 weeks to assess safety and efficacy of localized microinjection of TA for the treatment of melisma [6]. A total of 100 women with melasma, were enrolled and injected with 0.05ml of TA (4mg/mL) intradermally, weekly for 12 weeks. They observed significant decrease in the MASI from baseline to 8 and 12 weeks; side effects were minimal, and all the patients tolerated the treatment well.
Sharma et al. did a comparative study to ascertain the comparative efficacy of different routes of administration of TA [oral (250mg/ twice a day) and intradermal (4mg/mL every four week)] with sample size of 100 [7]. They found that both treatments routes were equally effective.
Samanthula et al. conducted a prospective study to assess the efficacy and side-effects of localized microinjection of TA for the treatment of melasma on 30 subjects [8] Patients were administered localized microinjections (10mg/mL) of TA weekly for 6 weeks. They concluded that increase in the dose (10mg/mL) and frequency of intralesional TA injections to once a week rather than a month showed good results without significant side effects.
Usual dose of oral TA for melasma is 500mg/day. Adverse events with oral TA therapy are listed as nausea, diarrhoea, malaise with hypotension, disturbances in colour vision, thromboembolic event, occasionally anaphylactic shock, and acute renal cortical necrosis. Localised microinjections allow lower doses of drugs to be used; hence, avoiding the side effects caused by oral route.
Our study results correspond with the related studies with minimal side effects. However, small sample size and single study site demands randomized controlled trials involving larger number of patients and longer duration follow up for further exploration of efficacy and safety of TA.
Based on the above findings, oral TA with antioxidants and local glycolic acid in combination can be used as a safe and effective therapeutic agent for the treatment of resistant melasma.
