Author(s): <p>Hong Yin*, Mathan Mohan Manoharan, Adam Truskewycz, Jim Jose, Daniel Lai and Ivan S Cole</p>
Using fluorescent quantum dots to detect heavy metal ions in water resources has attracted extensive attention. However, bulky photoluminescence spectrometers are generally required to read the signals, which are not suitable for on-site detection. This study designed a portable RGB-type quantum dot-based water sensing device using semiconductor quantum dots (SQDs) and carbon quantum dots (CQDs) as probes to detect various heavy metal ions. The results generated from the device in general agree well with those obtained from photoluminescence spectroscopy, demonstrating that the device caneffectively screen the presence of different heavy metal ions. Both the portable device and the photoluminescence spectrometer measurements confirmed that the SQDs used in this study could detect a lower concentration of heavy metal ions, but their fluorescence was quenched by a wide range of other ions at a higher testing concertation. In comparison, CQDs had better selectivity to Cadmium (Cd) ions. Our work verified the feasibility of the RGB-type quantum dot-based portable water sensing device, and the future work will focus on selecting a proper quantum dot material to achieve high selectivity and sensitivity simultaneously.
With the growth of industrialisation, pollution of heavy metal ions has become a major global concern because these contaminants are toxic, non-degradable and can accumulate in the environment. Currently heavy metal ions in water sources are detected by atomic absorption spectrometry (AAS), inductively couple plasma optical mass spectrometry (ICP-MS), and X-ray fluorescence spectroscopy (XRF). However, these techniques are not suitable for on-site detection because they require expensive and bulky instrumentation, tedious sample pre-treatment, and long processing time. The demand for accurate, robust and portable sensors and sensing devices for the detection and quantification of various heavy metal ions is increasing rapidly [1]. Water security and water quality are particularly issues in developing countries, and thus a low cost and easy-to-use device may assist in providing users vital information in these communities. The advantages of these portable sensing platforms over conventional instruments are low production cost, easy production, and low power consumption [2].
Fluorescent sensing using nanoparticle probes is an economical and easily miniaturized alternative for heavy metal sensing [3]. Quantum dots (QDs) have demonstrated unique properties, such as controlled fluorescence and photostability to enable them as promising sensing platforms for onsite detection of heavy metal ions [4]. Common mechanisms of QD-heavy metal ion response include photoinduced electron transfer (PET), metal to ligand charge transfer (MLCT), ligand to metal charge transfer (LMCT), intramolecular charge transfer (ICT), and fluorescence resonance energy transfer (FRET) [5]. Semiconductor QDs (SQDs) have various responses to different heavy metal ions because the affinity of these ions to the surface ligands on SQDs. Carbon quantum dots (CQDs), which are generally small carbon nanoparticles (less than 10 nm in size), have shown promising application for sensing heavy metals during the last few years [6]. Compared to SQDs, CQDs have the advantages of benign chemical composition, tunable fluorescence emissions, facile functionalization, and excellent physicochemical and photochemical stability [7]. The quenching or enhancing of fluorescence intensity of QDs may be caused by one or simultaneously two sensing mechanisms. Due to the complex of sensing mechanism, overwhelming reports show great performance in the detection of single heavy metal ions, however, comparing the response of SQDs and CQDs to various metal ions leaves a vast gap for future study.
To detect the fluorescence changes generated by a QD probe, the emitted fluorescence can be separated into a set of colours, primarily red (R), green (G), and blue (B). Data are frequently presented in terms of total colour differences (ΔC) using the Euclidean distance equation:

where ΔR, ΔG, and ΔB are the change in R, G, and B colours from reference values, respectively [8].
RGB colour model describes that most colours can be produced by varying the intensity values of three wavelengths corresponding to each primary colour, i.e., red, green, and blue. For example, black colour implies no light or zero intensity of each primary colour whereas white colour is produced by summing equal intensity of all three primary colours. Similarly, the yellow colour is an overlap of red and green intensities; magenta colour is the addition of red and blue intensities, and the cyan colour is generated by green and blue intensities. This principle forms the foundation of an RGB type sensing device to detect fluorescence changes generated by a QD probe.
In this study, a portable RGB-type sensing device based on SQDs and CQDs was designed for on-site detection of heavy metal ions. Seven different heavy metal ions were used as analytes. The workability of the device, including selectivity and sensitivity of SQDs and CQDs were detected, and the results were compared with those obtained from a fluorescence spectrophotometer.
Components in the Portable Sensing Device
The body of the portable device with a dimension of 7 cm x 10 cm x 5 cm was 3D-printed by an ANYCUBIC printer. It held the batteries, cuvette, circuitry, and optical filters. Three light emitting diodes (LEDs) with wavelengths of 375 nm, 405 nm, and 430 nm, respectively, were purchased from Thorlabs Inc. (Newton, USA) and used as excitation sources. A highly sensitive silicon photomultiplier (SiPM, TCS 3474 sensor) was used to detect corresponding fluorescent signals and transfer the signals to RGB values. An Arduino board (Beetle Bluno) was used in the device to enable Bluetooth 4.0 technology so that the RGB data could be transferred wirelessly to a smartphone. Figure 1 presents the schematic illustration of the RGB-type portable water sensing device.

Figure 1: Schematic Illustration of the RGB-Type Portable Water Sensing Device
Fluorescein solutions of various concentrations were prepared (0- 0.013 mg/mL) and placed in a cuvette. The solution in the cuvette was excited by the LED sources. The fluorescent emission was detected by the RGB sensor. Ten readings were taken for each solution with a sampling period of 100 ms. The data obtained from the 375 nm LED show the highest emission signals, and the average total colour differences (ΔC) were plotted against the concentrations of fluorescein with a good linear relationship (Figure 2). It suggested that the device with 375 nm LED excitation could detect ΔC accurately, which reflected the colour and the concentration of the fluorescein solution.
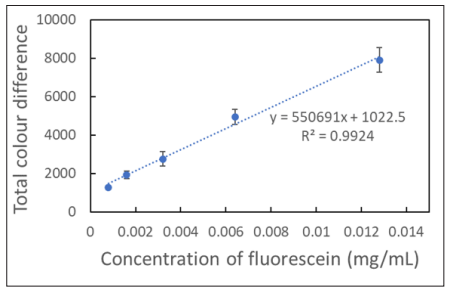
Figure 2: Correlation between the Total Colour Differences (ΔC) and the Concentration of Fluorescein
The QDs used in this study are listed in Table 1, which include glutathione-capped CdSe/CdS/ZnS SQDs and N-doped CQDs. These QDs have been reported for sensing different heavy metal ions. The preparation methods of these QDs are also described in Table 1.
|
Material |
Preparation method |
Reference |
|
Glutathione-capped CdSe/CdS/ZnS SQDs |
Cadmium oxide (1 mmol), oleic acid (18.85 mmol), and zinc acetate (2 mmol) were mixed with 1-octadecene and heated to 300°C under nitrogen. Then, selenium precursor (0.2 mmol) in trioctylphosphine (TOP) was added to the flask. After that, dodecanethiol was injected into the mixture to obtain CdSe/CdS SQDs. Subsequently, sulfur precursor in TOP with a concentration of 1 mmol was injected into the flask to obtain CdSe/CdS/ZnS SQDs. Finally, the SQDs were purified and re-dissolved in toluene. Water soluble CdSe/ CdS/ZnS SQDs were prepared by replacing the hydrophobic surface with glutathione. |
[9, 10] |
|
N-doped CQDs |
Sodium citrate (1.0 g) and polyacrylamide (0.52 g) were subjected to a hydrothermal reaction at 200 °C for 3 h. |
[11] |
Detection of Heavy Metal Ions by the Photolumine scence(PL) Spectrophotometer and the Portable Device
Seven heavy metal ions (Cd2+, Cr6+, Mn2+, Fe3+, Hg2+, Ni2+, and Pb2+) were used as analytes.
For the testing on the spectrophotometer, QD solution (1 mg/mL, 100 µL) was transferred into a 96-well plate with a clear glass bottom. Then, 100 µL of various metal ion solutions with a known concentration (0.1 or 1 ppm) were added into each well and mixed thoroughly. The resulting heavy metal ion concentration was 0.05 ppm or 0.5 ppm. The control well was supplemented with 100 µL of deionised water. The fluorescence spectra of the wells were recorded using a SpectraMax Paradigm Plate Reader (Molecular Devices) with an excitation wavelength of 360 nm.
To detect heavy metal ions using the portable device, any smartphone with a Bluetooth serial app (Handy BLE in this case) can be used to receive the results after paring with the device. Next, 2 mL of QD solution (1 mg/mL) was mixed with 18 mL of water to dilute the sensing probe. Then, 2 mL of diluted sensing QD solution was transferred to the cuvette and the RGB values were measured. After mixing 100 µL of 1 ppm heavy metal ion solution into the cuvette (final metal concentration 0.05 ppm), the RGB value was measured again. The processes were repeated after adding 100 µL of 10 ppm heavy metal ion solution in the same cuvette (final metal concentration 0.5 ppm).

Figure 3: (a) PL Spectra of SQDs in the Presence of 0.05 ppm Heavy Metal Ions, (b) PL Spectra of SQDs in the Presence of 0.5 ppm Heavy Metal Ions, (c) PL Spectra of CQDs in the Presence of 0.05 ppm Heavy Metal Ions, and (d) PL Spectra of CQDs in the Presence of 0.5 ppm Heavy Metal Ions.
As shown in Figure 3, the SQDs had a peak at 640 nm, which corresponded to its red emission color. When 0.05 ppm heavy metal ions were present, significant flouresence queching was observed for all ions; however, the spectra were similar for each type of heavy metal ions. When the heavy metal ion concnetration increased to 0.5 ppm, Ni, Fe, Cr, Hg and Cd ions induced significant queching, and Cd ions completedly queched the SQDs. In the same condition, when the SQDs were replaced by CQDs, no noticable queching occurred when 0.05 ppm heavy metal ions were present. When the concentration increased to 0.5 ppm, only Cd ions quenched the flouresence of CQDs, while the flouresence remained unchanged when the other metal ions were present.
The above results show that SQDs are more sensitive to heavy metal ions at a low concentration but with no selectivity. Whereas CQDs can only response to Cd at a relatively high concentration but have almost no quenching effect in the presence of the other ions.
First, the total colour difference of the blank QD solution without any heavy metal ions (ΔC0) was calculated using the generated R, G, and B values. Similarly, ΔCi, which is the total colour difference of the QD solution with specific heavy metal ions, was calculated. Fluorescence quenching ratio (QR) was calculated based on equation 2.

In a typical static quenching process, the interaction between heavy metal ions and QDs can change the environments of the QDs. The fluorescence quenching caused by the interaction between QDs and metal ions is a complicated process involving cation exchange, ligand detachment, electron transfer, surface ligand bonding and internal filtration quenching [12].
The QR values for SQDs and CQDs are shown in Figures 4 a and b, respectively. When the heavy metal concentration was 0.05 ppm, Cd ions induced significant fluorescence quenching in SQDs, while no quenching was observed in CQDs. With the concentration increasing to 0.5 ppm, more types of ions (Cr, Fe, Hg, and Ni) caused fluorescence quenching, and the QR was the highest for Cd. This was consistent with the spectrophotometer results. However, the QR for Cd ions only approached 0.85, while the quenching almost reached 100% in the PL spectrum.
For the CQDs, at the concentration of 0.5 ppm, the fluorescence was only quenched significantly when Cd ions were present, and the quenching ratio was about 0.8, which was similar as the highest peak intensity drop in Figure 3d.
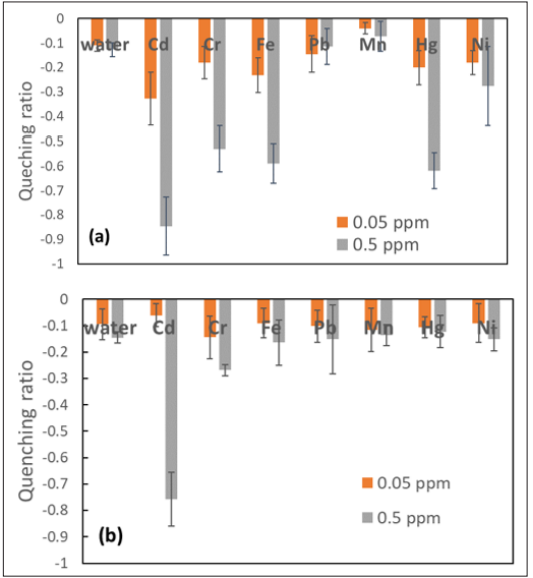
Figure 4: Quenching Ratio of (a) SQDs and (b) CQDs in the Presence of Various Heavy Metal Ions at 0.05 ppm and 0.5 ppm
The results obtained from the device agree well with those obtained from PL spectroscopy, demonstrating that the device can work properly to screen different heavy metal ions. In general, SQDs can detect lower levels of heavy metal ions, but without selectivity at a high testing concertation (Many types of metal ions can quench the fluorescence of SQDs to a significant level). In comparison, CQDs have better selectivity to Cd but can only detect it at 0.5 ppm.
By using SQDs and CQDs as probes for heavy metal ions, a portable RGB-type water sensing device was fabricated to detect various heavy metal ions. The fluorescence data of the QDs with or without the presence of heavy metal ions were obtained from PL spectroscopy and compared with those generated from the device. The comparison suggested that the portable device could effectively screen different heavy metal ions. The SQDs used in this study could detect a lower level of heavy metal ions, but its fluorescence was quenched by a wide range of ions at a high testing concertation. In contrast, CQDs had better selectivity to Cd ions with less sensitivity. The future work will focus on selecting a proper QD material to achieve high selectivity and sensitivity simultaneously.
