Author(s): Torri Clerici Valentina
Susac Syndrome (SuS) is a rare disease, characterized by an autoimmune endothelopathy, that involves brain, retina and inner ear. We describe a case of a young woman affected by inner ear-predominant SuS, initially suspected for demyelinating disease, with delayed diagnosis and irreversible hearing ischemic damage, but good prognosis, treated according to current recommendations.
Susac Syndrome (SuS) is a rare disease, characterized by an autoimmune endothelopathy that involves brain, retina and inner ear [1]. Its clinical manifestations are encephalopathy, branch retinal artery occlusion (BRAO), and low-frequency sensorineural hearing loss, but rarely this clinical triad is complete at first [1]. The natural disease course is highly variable for severity, extent, duration and prognosis, corresponding to the degree of microvasculopathy of involved organs. Severe cases might profit from prompt specific treatment.
When young patient present neurological symtoms and cerebral white matter lesions the most likely diagnosis is multiple sclerosis (MS), and SuS may not be recognized in particular when the clinical triad of SuS in incomplete at first. We describe a case of a young woman affected by inner ear-predominant SuS, with delayed diagnosis and irreversible hearing ischemic damage, but good prognosis, treated according to current recommendations.
A 22 year-old woman came to attention of our Multiple Sclerosis Centre in March 2019, for evaluating her brain MRI showing multiple cerebral white matter lesions, suspected for demyelinating disease. In the past three months, she had suffered from headache attacks with migraine characteristics preceded by visual and sensitive auras, which progressively increased in frequency. FANS, triptans, paracetamol, flunarizine and low-dose oral prednisone were associated with only partial benefit.
During hospitalization in May 2019, neurological examination was normal but she reported an acute right sensorineural hearing loss started two months before, treated with antibiotic therapy, without resolution. She also reported urinary urgency and mild cognitive dysfunction since the last year. Brain MRI showed T2 spotty focal lesions in the corpus callosum (CC), one of these with diffusion restriction, and small supra- and infratentorial punctate FLAIRhyperintense white matter lesions without restricted diffusion or pathological contrast enhancement (Figure.1). Spinal cord was uninvolved.
Cerebrospinal fluid (CSF) showed mildly increased protein count (60 mg/dl, normal range 10-45 mg/dl); intrathecal immunoglobulin synthesis was not detected. Autoimmune and thrombophylic screenings were unremarkable. The visual and the brainstem auditory evoked potentials were normal, but the audiogram showed a bilateral impairment of acoustic perception predominant on low frequencies. Automated perimetry revealed defect in both eyes (Figures 2a and 2b): on right eye the defect was predominantly in upper nasal area, on left eye in lower nasal area. Swept-source optical coherence tomography (SS-OCT) showed mildly increased thickness in both eyes (Figures 3a and 3b).
The diagnosis of Susac syndrome was retained following retinal fluorescein angiography (FAG), that showed acute hyperfluorescent areas in the right nasal superior areas on right eye, and ischemic lesion in the left nasal area on left eye (Figures 4a and 4b). The patient was treated with prednisone 75 mg daily for 3 days followed by tapering and ASA 100 mg daily for 14 days. One month following hospital discharge, she was admitted to the emergency department because of an acute sensorineural hearing loss in left ear, with impaired acoustic perception on low and middle frequencies. She was treated with methylprednisolone pulses (1 g/day for 5 days and 0.5 g for 3 days), and intravenous immunoglobulins-IVIg (120 g over 2 days: followed by 60 g every 4 weeks).
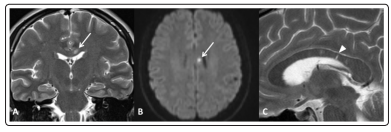
Figure 1: (A-Coronal T2wi) typical “snowball” lesion in corpus callosum with diffusion restriction (confirmed on ADC map, not shown) at high b-value in DWI (B)
(C-sagittal T2wi) After steroid therapy the main lesion disappeared but several thin lesions with radial orientation were visible, “spoke” lesions (arrowhead), deemed to represent old microinfarctions [2].
Figure 2: Automated perimetry / visual field
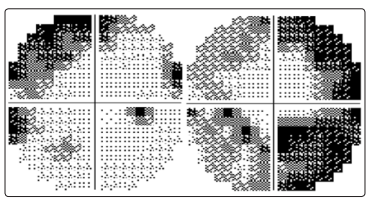
Figure 2a: upper nasal area visual field defect in right eye (MD -9.01), June 2019
Figure 2b: lower nasal area visual field defect in left eye (MD -15.62), June 2019
Figure 3: Swept-source optical coherence tomography (SSOCT)
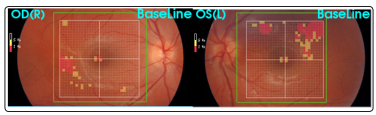
Figure 3a: SS-OCT, right eye (June, 2019)
Figure 3b: SS-OCT, left eye (June, 2019)
Figure 4: First Fluorescein angiography, June 2019
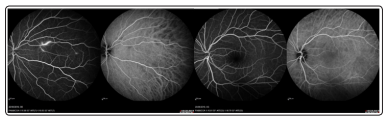
Figure 4a: acute hyperfluorescent areas in the right nasal superior areas on right eye
Figure 4b: ischemic lesion in the left nasal area on left eye
