Author(s): Nirali Patel and Vishwa Shukla*
The study aimed to investigate the performance of a fixed bed column using magnetic biochar as an adsorbent for synthetic dye wastewater treatment. The magnetic biochar was synthesized by pyrolyzing paper mill sludge at 350 ℃ and modified with iron oxide nanoparticles to enhance its magnetic properties. The adsorption capacity of the magnetic biochar was evaluated using methylene blue (MB) dye in wastewater. In present study the fixed bed column adsorption was conducted using MBC to treat MB wastewater. A fixed bed column setup was utilized in the experiments, maintaining a continuous flow rate of 15 mL/min at various bed height (i.e. 2.5, 5 & 7cm). Breakthrough & exhaustion capacity for each three different bed height at constant flow rate was also carried out. The Yoon-Nelson and Thomas kinetics models were also used in order to understand the dynamic performance of the fixed bed column. the respectively. The results shows that Yoon-Nelson model fitted best for the experimental data approximately R2 value of 0.96 against Thomas model R2 value of 0.95. Hence, it can be concluded that fixed bed column with MBC performed best with higher bed height & adsorption process is limited by film & pore diffusion.
Environmental pollution is a worldwide observable fact because of its unpleasant assets on the ecosystem and uncovered materials [1,2]. Huge extent of waste matter is discharged into the water bodies [1,3-5]. Synthetic dyes from textile, pharmaceutical, food, leather, and paint industries are consistently being released into water bodies [6]. Dyed wastewater from industrial processes pose civic and ecological risks such as aesthetic pollution due to their colour [7]. Most synthetic dyes, which are predominantly non-biodegradable and contain functional groups, are extremely toxic. Certain dyes within this category possess carcinogenic properties and can lead to skin and eye irritation as well [8]. These toxic dyes if not seperated from water bodies can generate a hazardous problem for mankind. Hence, these synthetic dyes must be removed from water bodies [9].
Dye wastewater treatment has utilized various biological, physical, and chemical techniques. These techniques encompass anaerobic/ aerobic treatment, coagulation/flocculation, oxidation/ozonation, membrane separation, and adsorption [10-16]. Adsorption is widely considered an effective technique for the decontamination of industrial discharges. Adsorption is favored primarily due to its cost-effectiveness in terms of design, land space, and operating expenses, as well as its superior efficiency when compared to alternative methods [17]. The adsorption technique does not leave behind by-products, the adsorbent can easily be separated from water, and are exceptionally effective for the removal of dyes [18- 22]. The effectiveness of the adsorption process primarily relies on factors like the adsorbent’s microporous structure, surface area, surface reactivity, and adsorption capacity, in addition to the operational conditions.
Silica gel, activated carbon, zeolite, and alumina are popularly employed in the adsorption process to eliminate impurities such as metal ions, toxic organic compounds, and harmful dyestuffs. [4,5,23-25]. Activated carbon is the most commonly used adsorbent for the adsorption of wastewater pollutants. Activated carbon is often favoured due to its high attraction for pollutants in wastewater, majorly because of its high surface area, microporous structure, high adsorption capacity, and high degree of surface reactivity.
In the adsorption system, there are several techniques through which the contact between the adsorbate and adsorbent primarily takes place, including batch processing, continuous moving bed, continuous fixed bed (up flow or down flow), continuous fluidized bed, and pulsed bed. [26,27]. Besides batch and fixed bed adsorption treatment, the remaining methods have certain disadvantages like requirement of large area, high cost, need for higher amount of adsorbent, feed channelling, pre-required adsorbent storage and non-uniform residence time [28].
Biochar, produced through the pyrolysis of biomaterials, is a promising adsorbent with a significant internal surface area and microporosity. It finds utility as an adsorbent in the separation and purification of gases, liquids, and colloidal solids [29-31]. In the literature, there have been reports of various studies conducted on the pyrolysis of paper sludge [32,33]. These studies aim to derive valuable products like biochar, biofuels, and adsorbents.
Researchers have utilized the resulting char from paper sludge pyrolysis as an adsorbent for applications in water treatment [34,35]. The adsorbents achieved from paper sludge have been used in the adsorption of metals, dyes, and organics from effluent [36]. Recently, magnetic biochar (i.e., modified biochar) adsorbents have been synthesized to enable easy recovery after the use [37-39] and used for adsorption of anionic contaminants such as Cr (VI), phosphate, and As (V) [40-42].
Batch adsorption studies give an important data and parameter on the removal of adsorbate, while column adsorption provide information on adsorption study with different of adsorbent used [43,44]. The limited experiment from the batch study can give the important information on the parameter used for modified in industry scale by fixed bed column [45,46]. In comparison to batch adsorption, continuous column is more advantageous as it is easy to operate, faster adsorption process and simplicity of scale up process [47]. The application of fixed bed adsorption is extensively employed in the purification of liquid mixtures, including those derived from industrial wastewater [48]. In recent studies, this technique has been investigated for the efficient removal of heavy metal effluents on a large scale [49].
Iron composites were incorporated into biochar (BC) through co-precipitation, using a 1:2 proportion of 3.66 gm FeSO4.7H2O and 6.66 gm FeCl3.6H2O distributed in 200 ml of distilled water. The mixture was stirred continuously for 1 hour to obtain Fe+2 and Fe+3. The pH was regulated to 10~11 by adding 1 N NaOH solution drop wise, followed by the addition of 10 gm of biochar. The subsequent suspension was stirred using a magnetic stirrer for 1 hour at 40 °C and left overnight to settle. Afterward, the suspension was dried for 2 hours at 105 °C.
Methylene blue is used to prepare synthetic dye wastewater which has similar characteristics of dye wastewater generating from dye industry. A 5000 mg/L methylene blue stock solution is prepared by dissolving 5 gm of methylene blue dye into 1000 mL distilled water.
A fied bed column was fabricated to carry out continuous column experiments. The column length was 50 cm and diameter 3 cm. The diagram of the column adsorption system is shown in Figure
1. The synthetic dye wastewater was pumped into the column with the help of an aquarium pump as shown in Figure 1.

Figure 1: Fixed Bed Column Experimental Set up with Different Bed Heights (2.5, 5 & 7 cm)
A flow regulator was used to regulate the flow rate, ensuring controlled movement. To prevent the loss of adsorbent during continuous operation, the magnetic biochar adsorbent was placed in the column with glass wool at the bottom. To examine the shape of the breakthrough curves under different operating conditions, samples were collected at 2-hour intervals until the adsorbents reached to exhaustion. The column study was conducted with variables bed heights of 2.5 cm, 5 cm, and 7 cm at constant flow rate of 15 mL/min.
The performance analysis of the fixed-bed column was conducted by assessing the dynamic behaviour through Breakthrough curves. The crucial factors for evaluating the column’s performance were the breakthrough time (t b ) and the characteristics of the different experimental conditions (including initial inlet dye concentration, Inlet Flow rate, pH of feed solution, and bed height), the breakthrough curve was generated by plotting the ratio of Ct (adsorbate concentration in the effluent) to C 0 (initial curve can be used to determine important parameters such as the breakthrough time, the adsorption capacity of the adsorbent, and the rate of adsorption. The breakthrough curve typically has an S-shaped or sigmoidal shape, with a rapid rise in concentration at the beginning of the curve. The shape of the breakthrough curve depends on the following parameters;
1. The properties of the adsorbent and the adsorbate,
2. The operating conditions of the column such as flow rate and concentration of the adsorbate in the feed stream
A well-designed adsorption system should have a breakthrough curve that is steep and narrow, indicating efficient adsorption with a high capacity and rapid rate.
The Yoon-Nelson and Thomas models are widely recognized models used in the modeling of continuous flow column reactors. These models were employed to analyze the dynamics of the methylene blue dye adsorption process in fixed-bed column configuration and make predictions regarding the breakthrough curves.
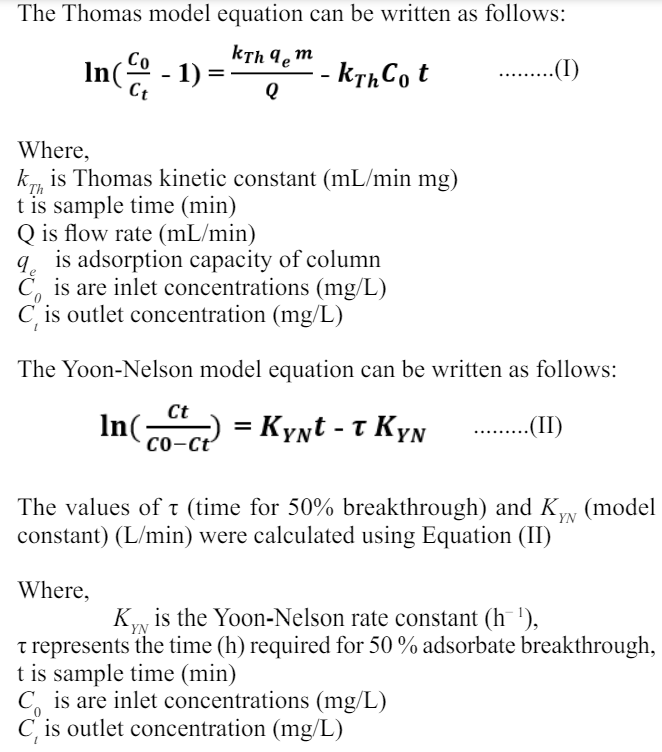
The fixed bed column study was carried out with methylene blue dye wastewater of 5000 mg/L at 15 mL/min flow rate with three bed heights of 2.5 cm, 5 cm and 7 cm respectively. The breakthrough curve for varying bed heights is expressed in Figure 2. It is observed that with the increase of bed height from 2.5 to 7 cm this causes an increase in breakthrough as well as exhaustion time. The rise in bed height and magnetic biochar leads to an increase in available adsorption sites, possibly explaining the reason behind this phenomenon. Additionally, the longer retention time of the effluent as it takes more time to reach the outlet results in an expansion of the total volume of treated effluent. The possible explanation for this phenomenon could be that a lower bed height affects the mass transfer process through axial dispersion, leading to a decrease in adsorbate diffusion. These findings suggest that a greater bed height holds the ability to effectively treat larger quantities of wastewater for extended durations. A similar trend was observed, where increasing the bed height resulted in longer exhaustion times and an increased total volume of treated effluent.
Figure 2: Effect of Bed Heights (2.5, 5 & 7 cm) on Fixed Bed Column Efficiency
Column Performance Breakthrough Point of Column Bed The critical moment in a fixed bed column occurs when the ratio between the concentration of the substance at the inlet and outlet increases from 0.05 to 0.9. At the breakthrough time, which happens when the ratio reaches 0.5, the adsorption capacity is determined. It is important to note that the column can continue operating until the ratio reaches 0.9, and the exhaustion time is determined when the concentration of the substance at the outlet is essentially identical to that at the inlet.
Table 2 includes different parameters of the fixed bed column at the breakthrough and exhaustion points as well as the column performance. At 2.5 cm bed height adsorption capacity of column was 2.4504 mg/gm, at 5 cm bed height adsorption capacity was 3.7254 mg/gm and at 7 cm bed height adsorption capacity was 5.5771 mg/gm.
The reason behind this phenomenon may be attributed to several factors. Firstly, the increased height of the bed allows for the treatment of a larger water volume and promotes the enhancement of the adsorption process. Additionally, the enlarged bed provides more adsorption sites and longer retention time, further improving the treatment capacity. Conversely, a lower bed height leads to faster saturation due to a reduced number of active sites, resulting in a rapid breakthrough point. Consequently, the removal efficiency is also increased. It has also been observed that with a lower bed height, saturation occurs more quickly, leading to higher removal efficiency.
Table 2 shows that the breakthrough time and saturation time increased with bed height rising from 120 to 1200 min, 360 to 2640 min, 1800 to 4800 min, respectively. Reducing the height of the bed decreases the mass of the bed, potentially leading to a smaller mass transfer zone and a slower diffusion rate of metal ions. In contrast, when comparing three different bed heights, the taller bed provides a larger mass transfer zone with an abundance of active sites. According to Table 2, a greater bed height broadens the mass transfer zone, leading to improved breakthrough and exhaustion times. As a result, a larger quantity of wastewater can be efficiently treated.
The paragraph explains that the process of mass transfer during adsorption is not only limited to chemical reactions and is affected by minimal diffusion resistance. In Figure 3, the Thomas model is presented, with the plot showing ln[(C0/Ct)-1] versus t, which allows for the determination of kTh and q0. The study’s regression coefficient, R2, indicates that the experimental data fits well when the height of the bed is increased. The values of kTh are -0.0025, -0.0026 & -0.2001 for bed height of 2.5, 5 & 7 cm respectively. For a bed height of 7 cm, the R2 value was 0.9767. This suggests that as the bed height increases, the value of k Th decreases while q 0 increases, which is consistent with previous findings.
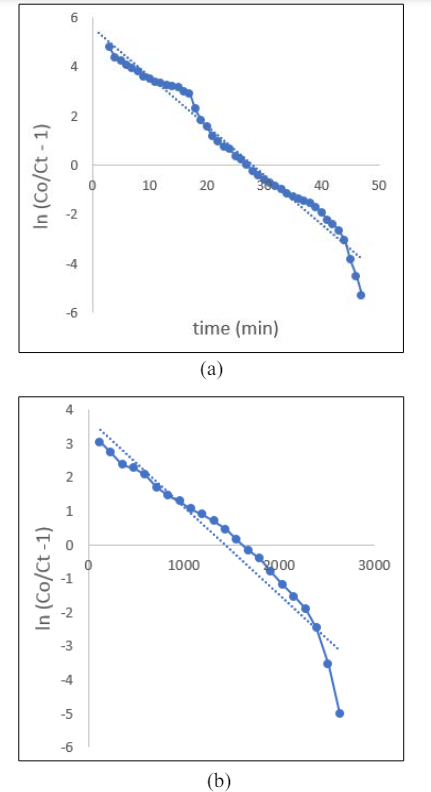
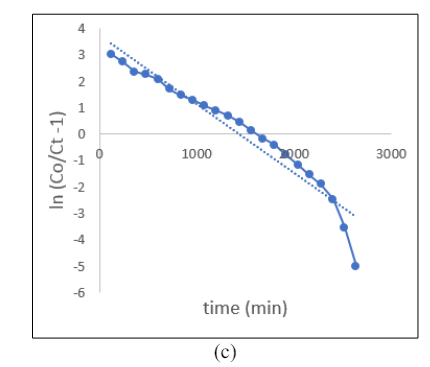
Figure 3: Thomas Isotherm for Bed Height (a) 2.5, (b) 5 and(c) 7 cm
When the bed height is set at 2.5 cm, 5 cm & 7cmt he values of KYN and t indicate that the 50% breakthrough time (t) increases as the bed height increases, while the rate constant, KYN, remains constant. The longer 50% breakthrough time signifies that adsorption will persist for a longer duration due to the presenceof a larger retention space. The regression coefficient, R2, indicates a good fit of the data set to the model (R2 ≥ 0.97). However, this model is more suitable for larger bed heights
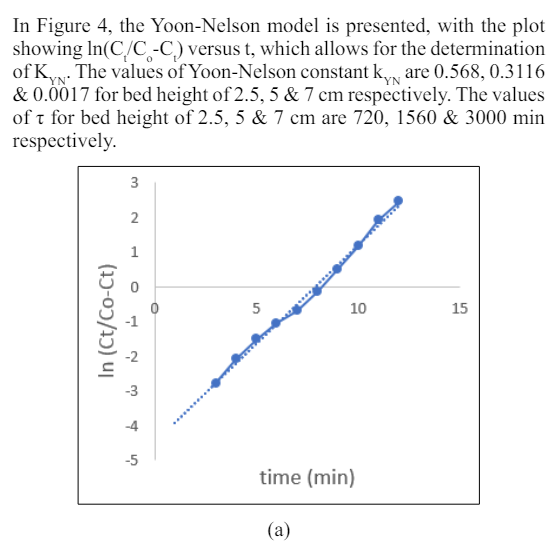
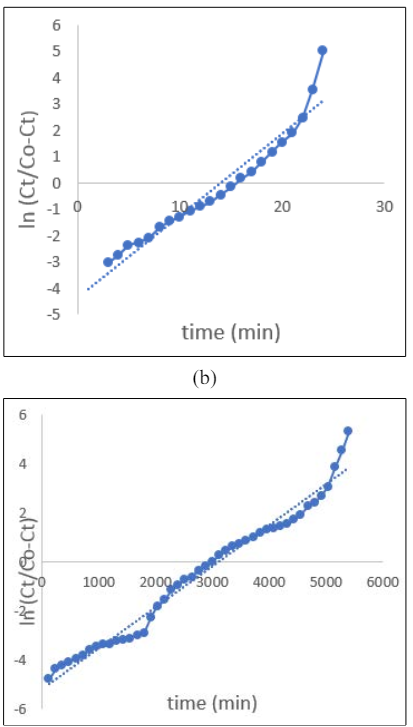
Figure 4: Yoon-Nelson isotherm for bed height (a) 2.5, (b) 5 &(c) 7cm
This study was carried out to investigate the use of fixed bed adsorption column to treat methylene blue dye wastewater using a magnetic biochar synthesised from paper mill sludge. According to the isotherm studies, results reveal that the Yoon- Nelson isotherm is the best model to illustrate the adsorption mechanism. The evaluation of fixed bed column performance involves numerous parameters, including flow rate, adsorbent quantity, and adsorbent particle size. The study findings indicate that increasing the adsorbent dose in the column improves the bed’s adsorbent capacity. Based on Thomas model results k Th (Thomas rate constant, mL/min mg) and qe (adsorption capacity,mg/gm) increases -0.0025, -0.0026 to -0.2001 & 2.4504, 3.7254 to 5.5771 for bed height 2.5, 5 and 7 cm. For Yoon-Nelson model k YN(Yoon-Nelson rate constant, h- 1) and t (50 % breakthrough 1800 for adsorbent bed height 2.5, 5 and 7 cm respectively. Yoon- Nelson model fitted best for the experimental data approximately R2 value of 0.96 against Thomas model R2 value of 0.95. Hence, it can be concluded that fixed bed column with MBC performed best with higher bed height & adsorption process is limited by film & pore diffusion.
1. David Noel S, Rajan MR (2014) Impact of Dyeing Industry Effluent on Groundwater Quality by Water Quality Index and Correlation Analysis. J Pollut Eff Control 2. https://doi. org/10.4172/2375-4397.1000126.
2. Ahmaruzzaman M, Gupta VK (2011) Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind Eng Chem Res 50: 13589-13613.
3. Asfaram A, Ghaedi M, Agarwal S, Tyagi I, Gupta VK (2015) Removal of basic dye Auramine-O by ZnS:Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Advances 5: 18438-18450.
4. Chakraborty V, Das P, Roy PK (2021) Graphene oxide–coated pyrolysed biochar from waste sawdust and its application for treatment of cadmium-containing solution: batch, fixed-bed column, regeneration, and mathematical modelling. Biomass Conv Bioref 13: 867-878.
5. Barman SR, Das P, Banerjee P, Mukhopadhayay A (2018) Urban wood waste as precursor of activated carbon and its subsequent application for adsorption of polyaromatic hydrocarbons. Int J Energy Water Res 2: 1-13.
6. Morsy S, Tajudinv A, Ali M, Shariff F (2020) Current development in decolorization of synthetic dyes by immobilized laccases. Front Microbiol 11: 572309.
7. Pathania D, Sharma S, Singh P (2013) Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab J Chem 10: S1445-S1451.
8. Pishnamazi M, Khan A, Kurniawan TA, Sanaeepur H, Albadarin AB, et al. (2021) Adsorption of dyes on multifunctionalized nano-silica KCC-1. Journal of Molecular Liquids 338: 116573.
9. Noor S, Taj M (2021) Mixed-micellar approach for enhanced dye entrapment: a spectroscopic study. Journal of Molecular Liquids 338: 116701.
10. Chinwetkitvanich S, Tuntoolvest M, Panswad T (2000) Anaerobic decolorization of reactive dyebath effluents by a two-stage UASB system with tapioca as a co-substrate. Water Research 34: 2223-2232.
11. Seshadri S, Bishop PL, Agha AM (1994) Anaerobic/ aerobic treatment of selected azo dyes in wastewater. Waste Management 14: 127-137. by powdered corn cob in batch and fixed bed system. Journalof Advanced Research 7: 79-87.
18. Gong R, Ye J, Dai W, Yan X, Hu J, et al. (2013) Adsorptive Removal of Methyl Orange and Methylene Blue from Aqueous Solution with Finger-Citron-Residue-Based Activated Carbon. Ind Eng Chem Res 52: 14297-14303.
19. Mittal H, Ballav N, Mishra SB (2014) Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of methylene blue from aqueous solution. Journal of Industrial and Engineering Chemistry 20: 2184-2192.
20. Musyoka SM, Mittal H, Mishra SB, Ngila JC (2014) Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet. International Journal of Biological Macromolecules 65: 389-397.
21. Aysan H, Edebali S, Ozdemir C, Karakaya MC, Karakaya N (2016) Use of chabazite, a naturally abundant zeolite, for the investigation of the adsorption kinetics and mechanism of methylene blue dye. Microporous and Mesoporous Materials 235: 78-86.
22. Chaukura N, Murimba EC, Gwenzi W (2016) Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge. Applied Water Science 7: 2175-2186.
23. Walker GM, Weatherley LR (2000) Prediction of bisolute dye adsorption isotherms on activated carbon. Process Safety and Environmental Protection 78: 219-223.
24. Tan C, Wang Q, Zhang CC (2011) Optical and electrochemical responses of an anthrax biomarker based on single-walled carbon nanotubes covalently loaded with terbium complexes. Chem Commun 47: 12521-12523.
25. Zhou Z, Wang Q, Wang J, Zhan CC (2015) Imaging two targets in live cells based on rational design of lanthanide organic structure appended carbon dots. Carbon 93: 671-680.
26. Kafshgari F, Keshtkar AR, Mousavian MA (2013) Study of Mo (VI) removal from aqueous solution: application of different mathematical models to continuous biosorption data. Iran J Environ Health Sci Eng 10: 14.
27. Miralles N, Valderrama C, Casas I, Martinez M, Florido A (2010) Cadmium and lead removal from aqueous solution by grape stalk wastes: modeling of a fixed-bed column. J Chem Eng Data 55: 3548-3554.
28. Singh VP, Yadava RN (2003) Wastewater Treatment and Waste Management; Proceedings of the International Conference on Water and Environment (WE-2003), December 15-18, 2003, Bhopal, India. https://www.amazon.com/Wastewater-Management-Proceedings-International-Environment/dp/8177645447
29. Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, et al.(2013) Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol 130: 457-462.
30. Alslaibi TM, Abustan I, Ahmad MA, Foul AA (2014) Preparation of Activated Carbon From Olive Stone Waste: Optimization Study on the Removal of Cu2+, Cd2+, Ni2+, Pb2+, Fe2+, and Zn2+ from Aqueous Solution Using Response Surface Methodology. J Dispers Sci Technol 35: 913-925.
31. Gwenzi W, Musarurwa T, Nyamugafata P, Chaukura N, Chaparadza A, et al. (2014) Adsorption of Zn(2+) and Ni(2+) in a binary aqueous solution by biosorbents derived from sawdust and water hyacinth (Eichhornia crassipes). Water Sci Technol 70: 1419-1427.
32. Mendez A, Barriga S, Saa A, Gabriel Gascó (2010) Removal of malachite green by adsorbents from paper industry waste materials. Journal of Thermal Analysis and Calorimetry 99: 993-998.
33. Cho R, Suzuki M (1980) Activated carbon by pyrolysis of sludge from pulp mill wastewater treatment. Journal of Chemical Engineering of Japan 13: 463-467.
34. Khalili NR, Vyas JD, Weangkaew W, Westfall SJ, Parulekar SJ, et al. (2002) Synthesis and characterization of activated carbon and bioactive ad sorbent produced from paper mill sludge. Separation and Purification Technology 26: 295-304.
35. Vamvuka D, Salpigidou N, Kasanaki E, Sfakiotakis S (2009) Possibility of using paper sludge in co-firing applications. Fuel 88: 637-643.
36. Chen B, Chen Z (2009) Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 76: 127-133.
37. Yang JP, Zhao YC, Ma SM, Zhu BB, Zhang JY, et al. (2016) Mercury removal by magnetic biochar derived from simultaneous activation and magnetization of sawdust. Environ Sci Technol 50: 12040-12047.
38. Sun PF, Hui C, Khan RA, Du JT, Zhang QC, et al. (2015) Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci Rep 5:12638.
39. Yan LL, Kong L, Qu Z, Lo L, Shen GQ (2015) Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sust Chem Eng 3: 125-132.
40. Wang SY, Tang YK, Li K, Mo YY, Li HF, et al. (2014) Combined performance of biochar sorption and magnetic separation processes for treatment of chromiumcontained electroplating wastewater. Bioresour Technol 174: 67-73.
41. Zhou YM, Gao B, Zimmerman AR, Chen H, Zhang M, et al. (2014) Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour Technol 152: 538-542.
42. Zhang F, Wang X, Ji XH, Ma LJ (2016) Efficient arsenate removal by magnetitemodified water hyacinth biochar. Environ Pollut 216: 575-583.
43. Ahmad AA, Hameed BH (2010) Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. Journal of Hazardous Materials 175: 298-303.
44. Dutta M, Basu JK (2013) Fixed-bed Column Study for the Adsorptive Removal of Acid Fuchsin Using Carbon– alumina Composite Pellet. International Journal of Environmental Science and Technology 11: 87-96.
45. Cybelle Morales Futalan, Chi-Chuan Kan, Maria Lourdes Dalida, Chelo Pascua, Meng-Wei Wan (2011) Fixed-bed column studies on the removal of copper using chitosan immobilized on bentonite. Carbohydrate polymers 83: 697- 704.
46. Mulgundmath VP, Jones RA, Tezel FH, Thibault J (2012) Fixed bed adsorption for the removal of carbon dioxide from nitrogen: breakthrough behaviour and modelling for heat and mass transfer.Separation and purification technology 85: 17-27.
47. Gaikwad RW (2013) Removal of copper (II) ions from Acid Mine Drainage effluents using Psidium Guava leaves powder. Int J Adv Res 1: 944-950.
48. Yelebe ZR, Yelebe BZ, Samuel RJ (2020) Design of fixed bed column for the removal of metal contaminants from industrial wastewater. J Eng Appl Sci 5: 68-77.
49. Babu BV, Suresh Gupta (2004) Modeling and simulation for dynamics of packed bed adsorption. In Proceedings of international symposium & 57th annual session of IIChE in association with AIChE (CHEMCON-2004), Mumbai December 27-30.
View PDF