Author(s): Antony Macido, DNP, ACNP-BC, CNS
Background: Sepsis continues to be one of the most frequent causes of mortality in critically ill patients globally. Septic cardiomyopathy or sepsis induced cardiomyopathy (SICM) is a phenomenon that is often undiagnosed and underdiagnosed in septic states. The significance of appropriate identification and management of SICM in the prognosis and outcomes of sepsis remain unclear.
Aim: This paper provides a brief review of the incidence of SICM and its appropriate diagnosis in septic states. The paper also provides an insight into the pathophysiology of SICM and the strategies employed in the clinical management of this entity.
Methods: A brief umbrella review was conducted on the incidence and diagnosis of SICM including its pathophysiology. The paper also reviewed the current evidence supporting the appropriate identification and management of SICM including any available guidelines employed in the management of it.
Findings: There is no universally agreed definition of SICM. The pathophysiology of SICM is intricate and its diagnosis and management remain complex. No specific guidelines are available to date in aiding the diagnosis and management of SICM.
Conclusion: Despite being a reversible phenomenon, SICM can increase the mortality associated with sepsis. Appropriate identification and management of SICM may have prognostic implications in sepsis and septic shock.
Sepsis is a common clinical diagnosis and the definition of sepsis keep on evolving over the years. Sepsis implies a life-threatening organ dysfunction resulting from a dysregulated host response to an infection. Organ dysfunction in sepsis can be clinically defined as an increase in the Sequential Organ Failure Assessment (SOFA) score by 2 or more points. A SOFA score of ≥2 can reflect an approximate 10% mortality risk in patients with suspected infection in a hospital. Sepsis could culminate in septic shock that is defined as profound metabolic, cellular, and circulatory dysregulation from underlying sepsis. Septic shock is clinically diagnosed as a need for vasopressors to keep a mean arterial pressure of 65 mm Hg or more and lactic acidosis greater than 2 mmol/L (>18 mg/dL) along with no evidence of underlying hypovolemia. Meeting the septic shock criteria increase the inpatient mortality rate to more than 40% [1].
Sepsis is associated with significant mortality rates and economic impacts. It is estimated that approximately 1.7 million adults in the United States develop sepsis annually and approximately 350,000 adults who develop sepsis die during that hospitalization. This translates into 1 in 3 individuals with a diagnosis of sepsis ending up dying during the respective hospitalization [2]. Despite advances in medical sciences, sepsis continues to be one of the most frequent causes of mortality in critically ill patients globally [3]. Sepsis is also an expensive diagnosis, costing roughly $62 billion annually for hospitalization and skilled nursing care [4]. Sepsis increases the risk of developing sepsis induced cardiomyopathy (SICM), a condition that can further increase the mortality associated with sepsis [5,6]. SICM is an entity that is often undiagnosed or underdiagnosed. SICM reportedly occurs in approximately 10% to 70 % of septic patients [7].
This paper provides a brief review of the incidence of SICM and the appropriate identification of the phenomenon in septic states. The paper also provides an insight into the pathophysiology of SICM and the strategies employed in the clinical management of this entity.
The terms sepsis induced cardiomyopathy (SICM) and sepsis induced myocardial dysfunction (SIMD) essentially imply the same clinical entity. For ease of reference, the term SICM is used in the remainder of the article. SICM is essentially a nonischemic and rather temporary cardiac dysfunction that can ensue during sepsis. Despite extensive literature available on SICM, an objective definition has not been established. But expert opinion and literature review suggests that SICM is a newly identified decline in left ventricular ejection fraction (LVEF) of </= 50% or a 10% decline in LVEF in patients with a known history of heart failure with reduced ejection fraction (HFrEF) [5]. Other characteristic features suggested as diagnostic of SICM include acute onset and reversibility (often in 7 to 10 days) and no identified acute coronary syndrome as the etiology. It is also proposed that SICM often results in biventricular dysfunction (systolic and/or diastolic) and dilated left ventricle along with poor response to volume resuscitation [8].
Literature search was done using major electronic databases including but not limited to Cochrane Library, ProQuest, PubMed Central, and Medical Literature On-Line. The inclusion criteria were systematic reviews, retrospective studies, observational studies, case studies, and guidelines related to sepsis, septic shock, and SICM. Key search terms used included sepsis; septic shock; diagnosis of sepsis and septic shock; management of sepsis and septic shock; septic cardiomyopathy; sepsis induced cardiomyopathy; sepsis induced myocardial dysfunction; incidence of septic cardiomyopathy; diagnosis of septic cardiomyopathy; pathophysiology of septic cardiomyopathy; management of septic cardiomyopathy; guidelines for management of septic cardiomyopathy; and vasopressors and ionotropes for septic cardiomyopathy. The Boolean operators such as “AND,” and the truncation symbol asterisk was also employed for searching the articles. The search criteria were refined to full-text and peerreviewed articles. The search criteria involved only publications in English. Once the appropriate articles were identified, the results of the studies were reviewed to arrive at conclusions as described in the section below.
The pathophysiology of SICM is intricate and is suggested to have activation of inhibitors of myocardium such as interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor α (TNF - α). SICM also involves compliment system activation, mitochondrial dysfunction involving production of reactive oxygen species (ROS) and nitric oxide (NO), alterations in calcium homeostasis, and other complex mechanisms [9,10]. The immune cells of the body recognize components of pathogens referred to as pathogen associated molecular patterns (PAMPs) with special receptors named pattern recognition receptors (PRRs). Cellular damage from PAMPs cause release of various endogenous molecules and cytokines known as damage-associated molecular patterns (DAMPs). DAMPs and PAMPs bind to PRRs with resultant activation of the respective signal pathways and produce cytokines including TNF - α and interleukin - 1β (IL-1β). These cytokines eventually cause myocardial dysfunction. Proton gradient (H+) entry into the mitochondrial matrix is caused by uncoupling proteins (UCPs) instead of adenosine triphosphatase (ATPase), leading to uncoupling of ATP production and eventually causing myocardial dysfunction. Opening of the calcium channels on the myocardial cell membrane with sepsis results in influx of large amounts of calcium into the cytoplasm that results in opening of mitochondrial permeability transition pore (mPTP) on the mitochondria so that cytochrome C and mitochondrial DNA (mtDNA) enter the cytoplasm with eventual cell apoptosis. NO production by L-arginine along with the influence of nitric oxide synthase (NOS) combines with superoxide O2 forming toxic peroxynitrite anion (ONOO-), which results in oxidative stress and eventual myocardial dysfunction [11]. Please see figure 1.
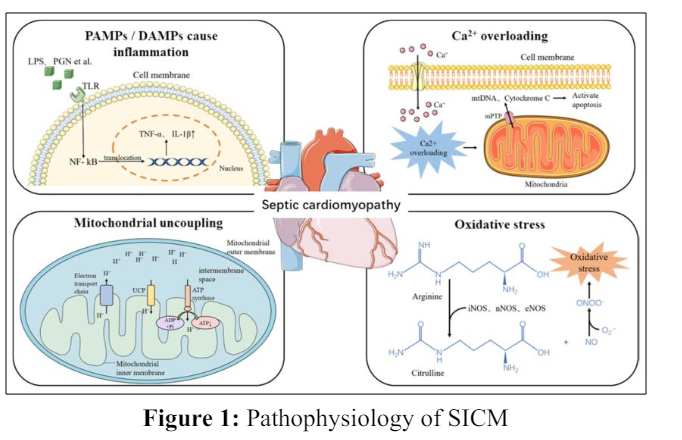
Abbreviations: DAMPs, damage- associated molecular patterns; IL-1β, interleukin 1β; LPS, lipopolysaccharide; mPTP, mitochondrial permeability transition pore; mtDNA, mitochondrial DNA; NF-kB, nuclear factor kB; NO, nitric oxide; NOS, nitric oxide synthase; O2-, superoxide; PAMPs, pathogen-associated molecular patterns; PGN, peptide polysaccharides; TNF-α, tumor necrosis factor α; UCP, uncoupling protein.
There are no electrocardiogram (EKG) changes or any biomarkers that are specific for the diagnosis of SICM. Transthoracic echocardiography (TTE) is the cornerstone diagnostic tool for identifying SICM [8]. TTE is a relatively inexpensive, easily available, and non-invasive diagnostic tool that is frequently used in Critical Care settings. Estimation of LVEF is a poor diagnostic tool for identifying SICM as LVEF estimation is influenced by afterload and preload conditions. LVEF estimation can change remarkably and quickly based on the volume status and mean arterial pressures that may be rapidly shifting in septic states [12,13]. As such, identification of reduced contractility with speckle tracking echocardiography (STE), irrespective of the LVEF is the diagnostic approach supported by evidence [8]. STE reflects myocardial motion by ultrasonographic echo tracking in the cardiac muscles during the cardiac cycle and is frequently reported as global longitudinal strain (GLS). GLS represents contractility and is calculated by measuring the differences between the final length and length at rest of the myocardium [14]. Normal GLS values may be somewhat different based on the software and the vendor. The American Society of Echocardiography considers a GLS around -20% as the normal, with less negative values reflecting decreased contractility [15]. Additional diagnostic modalities not altered by rapidly altering hemodynamic parameters in septic states (preload, afterload, and heart rate) using TTE that are used to evaluate SICM are supported by literature. Myocardial performance index (MPI) sometimes known as Tai index evaluates the myocardial function independent of preload and heart rate (that could be rapidly changing in septic states). A prospective study that used MPI to evaluate cardiac function in septic states showed that lower MPIs were associated with better prognosis in SICM [16].
Sepsis alone is an important cause of mortality and the presence of SICM has prognostic ramifications in the care of these patients.
There are no studies that define the treatment goals in SICM. As such, comprehensive work-up and management of individual patients need to be employed to improve outcomes in patients with SICM. Fluid challenge or fluid resuscitation is one of the initial management of patients in sepsis and septic shock. There is increasing evidence that over resuscitation with fluids and positive fluid balance can have detrimental effects in sepsis and septic shock. The SOAP trial demonstrated that a positive fluid balance was strongly associated with poor prognosis including death in septic patients [17]. The detrimental effects of over resuscitation are more likely to be pronounced in SICM. As such, judicious use of fluids after the initial resuscitation phase and assessing the fluid responsiveness with dynamic measures of fluid responsiveness such as inferior vena cava (IVC) assessment, pulse pressure variation, and passive straight leg raise are recommended [8].
The latest guidelines for the management of sepsis and septic shock recommend norepinephrine as the first-line agent for septic shock. The guidelines suggest the addition of dobutamine to norepinephrine or using epinephrine alone in patients with cardiac dysfunction accompanied by septic shock and hypoperfusion despite adequate volume status and arterial blood pressure [18]. The pathophysiology of sepsis and SICM involves increasing NO production, thus inducing vasodilation and decreasing the response to catecholamines. Methylene blue (MB) that limits NO production by inhibiting guanylate cyclase and NOS is often used to treat vasoplegia following cardiovascular surgeries. The use of MB to decrease the effects of NO in sepsis and SICM seems to be a potential option. Although MB use in sepsis and septic shock has shown decreased pressor requirements, studies have shown no significant difference in other outcomes [19]. Although MB is a potential treatment for septic shock, evidence on the use of MB in SICM is lacking. Since the pathophysiology of SICM involves alterations in calcium homeostasis in the myocardial cells, levosimendan seems to be a potential agent that can be used in SICM. Levosimendan (not available in the United States) provides positive inotropic effect by binding to cardiac troponin C in a calcium-dependent fashion, making myofilaments sensitive to calcium [20]. Despite the continuing use of dobutamine and levosimendan in cardiovascular failure associated with sepsis in critically ill patients, there is controversial evidence on the superiority of one over the other in the management of SICM. One of the first available meta-analysis that compared the effectiveness of dobutamine and levosimendan in SICM reported levosimendan use with better improvement in left ventricular stroke work index (ΔLVSWI) (random effects, SMD=1.56 [0.90,2.21]; I2=65%, P=0.04) and cardiac index (ΔCI) (random effects, SMD=0.90 [0.20,1.60]; I2=76%, P<0.01) when compared to dobutamine. Levosimendan use was also associated with significantly improved clearance of blood lactic acid (Δblood lactate) (random effects, MD=-0.79 [-1.33,-0.25]; I2=68%, P<0.01) [21]. However, the Surviving Sepsis Guidelines recommend against the use of levosimendan in adults with septic shock and cardiac dysfunction with persistent hypoperfusion despite appropriate arterial blood pressure and adequate volume status [18]. The Surviving Sepsis Guidelines do not provide any recommendations on the use of milrinone in SICM.
Mechanical circulatory support (MCS) devices including intraaortic balloon pump (IABP), percutaneous ventricular assistive devices such as Impella®, and/or extracorporeal membrane oxygenation (ECMO) are frequently employed in refractory cardiogenic shock from underlying congestive heart failure (CHF). Many case reports are available on the successful use of MCS devices in SICM [22]. The utilization of MCS devices for SICM is based on their utility in refractory cardiogenic shock associated with CHF. There are no controlled studies or large studies on the utilization of MCS devices in SICM. As such, the use of MCS devices need to be reserved in extreme cases of SICM [8].
SICM is a phenomenon that is probably undiagnosed and underdiagnosed in Critical Care settings. Prompt diagnosis and subsequent management of SICM probably has implications on the prognosis and subsequent mortality in septic states. Appropriate diagnosis of SICM can be challenging given the various proposed criteria for diagnosis as well as the lack of a universally agreed definition. TTE measurements that are not affected by preload and afterload conditions (such as GLS) if available should be considered for the diagnosis of SICM. Once SICM is suspected or confirmed, judicial use of fluids versus aggressive fluid resuscitation needs to be employed while employing dynamic measures of fluid responsiveness to confirm adequate resuscitation. For patients with suspected or confirmed SICM who are already on norepinephrine for underlying septic shock, addition of dobutamine can be considered if there is evidence of hypoperfusion despite adequate volume status and arterial blood pressure. Although, dobutamine increases tissue oxygen delivery (DO2) and improves perfusion, there has been reported evidence of increased 90-day mortality with the use of dobutamine in septic shock [23]. For patients with underlying SICM, with evidence of hypoperfusion despite adequate fluid resuscitation and adequate arterial pressure, epinephrine could be considered as the vasopressor of choice. Although not recommended by the latest Surviving Sepsis Guidelines, levosimendan (if approved for clinical use locally) can be considered as an appropriate alternative to dobutamine. In extreme cases of septic shock and SICM refractory to vasopressors, measures need to be taken for early initiation of MCS devices versus referral to facilities capable of provision and support of MCS devices. Table 1 could be used as an algorithm to aid in the clinical management of SICM in Critical Care settings.
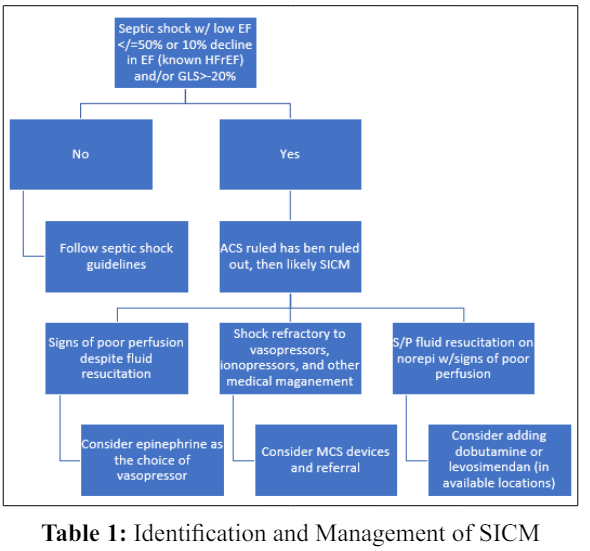
Sepsis and septic shock are considered medical emergencies that require prompt and appropriate management [18]. SICM is an undiagnosed or underdiagnosed phenomenon that often accompanies septic shock. Despite the reversible feature of SICM, the condition is associated with high mortality. Therefore, prompt recognition and appropriate management of SICM are very essential in sepsis states. TTE is the preferred diagnostic tool for the diagnosis of SICM. Appropriate identification and management of SICM may have prognostic implications in sepsis and septic shock. The treatment goals for SICM are not well defined. No specific guidelines are available to date in aiding the diagnosis and management of SICM. There is a scarcity of literature to recommend the best evidence-based practice in the diagnosis and management of SICM. Further research is needed to better understand the pathogenesis and management of SICM.
